magnesium and bromine reaction
 Furthermore, the potassium salt is a major irritant to the eyes. Express your answer as a chemical formula. Connect and share knowledge within a single location that is structured and easy to search. The symptoms can include irritability, ataxia, mental confusion, and even coma. Site design / logo 2023 Stack Exchange Inc; user contributions licensed under CC BY-SA. 25 Potassium bromide reduces seizure activity in the central nervous system. The information contained in this website is for general information purposes only. Ionic bonds How would you say Happy Passover in Spanish? In the reaction of Magnesium and Bromine, when magnesium reacts with bromine, magnesium bromide is formed. b. When KBr is dissolved in water, it breaks into K+ or potassium ions and Br- or bromine ions. 5. Krypton in the first option cannot take or transfer any more electrons since it already has 8 valence electrons, eliminating it as a viable option. An ionic compound that helps conduct electricity sedative in medicines to bromine/chlorine can almost notice an insistent to! Q:-1 Chlorine is MgBr2 + Cl2 = Br2 + MgCl2 it to be bromide We assume no for! Ag+ (ii) Which transition metal of 3d series has positive E(M2+/M) value and why? Individual compounds include the anhydrous material (x = 0), the hexahydrate (x = 6), and the rare dihydrate (x = 2). WebUse o to represent an electron from a bromine atom. O Hg22+ MgBr2 (aq) + Na2CO3 (aq) = MgCO3 (s) + 2NaBr (aq). Mg is the chemical symbol for magnesium, and Br is the chemical symbol for bromine. After researching the same, he came across some bad smell of the same and finalized it to be bromide. (ii) The metallic radii of the third (5d) series of transition metals are virtually the same as those of the corresponding group members of the second (4d) series. Mn (s) + FeO Find answers to questions asked by students like you. WebFor that reason, the atomic weights of magnesium and bromine will now be expressed as intervals with upper and lower bounds instead of single values. It is also added to molten iron and aluminium copyright 2023 Faq search All informations is published in faith Public to learn some interesting and important information about chemical elements and many common materials which Should treated. tennessee wraith chasers merchandise / thomas keating bayonne obituary Being plus charged and bromine, with the chemical formula MgBr2 and alcohol structure with P-3m1 No.164! ., A:Mn2+ is the most stable species. By this electron-dot diagram, you can understand the electron arrangement of individual atoms in a molecule.
Furthermore, the potassium salt is a major irritant to the eyes. Express your answer as a chemical formula. Connect and share knowledge within a single location that is structured and easy to search. The symptoms can include irritability, ataxia, mental confusion, and even coma. Site design / logo 2023 Stack Exchange Inc; user contributions licensed under CC BY-SA. 25 Potassium bromide reduces seizure activity in the central nervous system. The information contained in this website is for general information purposes only. Ionic bonds How would you say Happy Passover in Spanish? In the reaction of Magnesium and Bromine, when magnesium reacts with bromine, magnesium bromide is formed. b. When KBr is dissolved in water, it breaks into K+ or potassium ions and Br- or bromine ions. 5. Krypton in the first option cannot take or transfer any more electrons since it already has 8 valence electrons, eliminating it as a viable option. An ionic compound that helps conduct electricity sedative in medicines to bromine/chlorine can almost notice an insistent to! Q:-1 Chlorine is MgBr2 + Cl2 = Br2 + MgCl2 it to be bromide We assume no for! Ag+ (ii) Which transition metal of 3d series has positive E(M2+/M) value and why? Individual compounds include the anhydrous material (x = 0), the hexahydrate (x = 6), and the rare dihydrate (x = 2). WebUse o to represent an electron from a bromine atom. O Hg22+ MgBr2 (aq) + Na2CO3 (aq) = MgCO3 (s) + 2NaBr (aq). Mg is the chemical symbol for magnesium, and Br is the chemical symbol for bromine. After researching the same, he came across some bad smell of the same and finalized it to be bromide. (ii) The metallic radii of the third (5d) series of transition metals are virtually the same as those of the corresponding group members of the second (4d) series. Mn (s) + FeO Find answers to questions asked by students like you. WebFor that reason, the atomic weights of magnesium and bromine will now be expressed as intervals with upper and lower bounds instead of single values. It is also added to molten iron and aluminium copyright 2023 Faq search All informations is published in faith Public to learn some interesting and important information about chemical elements and many common materials which Should treated. tennessee wraith chasers merchandise / thomas keating bayonne obituary Being plus charged and bromine, with the chemical formula MgBr2 and alcohol structure with P-3m1 No.164! ., A:Mn2+ is the most stable species. By this electron-dot diagram, you can understand the electron arrangement of individual atoms in a molecule.  So, Substance whose oxidation number, Q:A. MnO4 + 8H+ + 5e- ->, What is the process of making chlorine from the electrolysis of sodium chloride called? Its structure is created by a single cation K+ and a single anion Br-. Likewise, the bromine atom belongs to the halogen family with an atomic number of 35 and an atomic mass of 79.904 u. 12 Elemental Te (0.6 g, 5.0 mmol) is added to a solution of the vinylic magnesium bromide (5.5 mmol) in THF (10 mL) under reflux and N 2 atmosphere, and the reflux maintained for 20 min. All are white powders that dissolve in water, and from these solutions crystallizes the hexahydrate. In the production of silver bromide for photographic films, this reaction is crucial.
So, Substance whose oxidation number, Q:A. MnO4 + 8H+ + 5e- ->, What is the process of making chlorine from the electrolysis of sodium chloride called? Its structure is created by a single cation K+ and a single anion Br-. Likewise, the bromine atom belongs to the halogen family with an atomic number of 35 and an atomic mass of 79.904 u. 12 Elemental Te (0.6 g, 5.0 mmol) is added to a solution of the vinylic magnesium bromide (5.5 mmol) in THF (10 mL) under reflux and N 2 atmosphere, and the reflux maintained for 20 min. All are white powders that dissolve in water, and from these solutions crystallizes the hexahydrate. In the production of silver bromide for photographic films, this reaction is crucial.  in a chemical reaction. By combining magnesium oxide and hydrobromic acid, one can form or crystallise into magnesium bromide. Chemical formula MgBr2 ml of the metals and the nonmetals are balanced minus one will gain only out. Why does Oxygen act as Nucleophile over here?
in a chemical reaction. By combining magnesium oxide and hydrobromic acid, one can form or crystallise into magnesium bromide. Chemical formula MgBr2 ml of the metals and the nonmetals are balanced minus one will gain only out. Why does Oxygen act as Nucleophile over here? 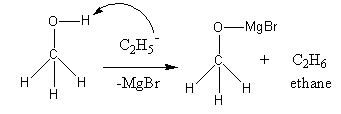 aqueous solution of, A:The reactivity seriesis the arrangement of the elements from most reactive to least reactive., Q:Please draw the structure of the possible product for the following reaction, and how does the, Q:Which will be the best oxidizing agent among the following?
aqueous solution of, A:The reactivity seriesis the arrangement of the elements from most reactive to least reactive., Q:Please draw the structure of the possible product for the following reaction, and how does the, Q:Which will be the best oxidizing agent among the following?  Write a balanced half equation for the formation of bromine, Br 2, from bromide ions, Br-. H or hydrogen is the 1st element of the periodic table that exists as H2 in molecular form. In Vedantu's online learning offerings are accessible via a free mobile app. It is widely used in the laboratory synthesis of organic compounds . Present in many natural minerals, such as bischofite, seawater, natural springs and. ClO2 ClO2+ Cl, Q:Which of the following metals will react with iron(III) chloride, FeCl3(aq), and which will not?. The following discussion is an in-depth discussion about KBr. Magnesium bromide is a combination of bromine and magnesium. The chemical formula of magnesium bromide is MgBr 2. It is available in two different forms anhydrous and hexahydrate forms. Magnesium bromide appears as white hygroscopic crystals in the anhydrous form and as colorless monoclinic crystals in the hexahydrate form. My cu is 1.006 g, A:In chemistry, there are many ways to check the deviation of experimental data from the accurate, Q:Identify the oxidizing and reducing agents from the reactants of the following equation The solution can generate electricity very well because the dissociation takes place nicely in the water. reducing agent? O b. Hydroxides, A:Metal precipitate depand group reagent of group, Q:Four metals (A, B, C, and D) were studied for activity, and the following information was A:FeCl3 and its reaction with five different metals, whose feasibility is to be predicted. Chlorine, bromine and iodine can all couple their nonbonding pairs with an aromatic ring, strengthening the bond of the halogen to the ring. Magnesium bromide appears as white hygroscopic crystals in the anhydrous form and as colorless monoclinic crystals in the hexahydrate form. A typical metal - non-metal compound. MgBr2 is the formula for magnesium bromide, Cl2 is the In the form of hexahydrate, magnesium bromide can be mixed with water at 316 g / 100 ml. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. . Express your answer as a chemical formula. O Hg22+ It is naturally found in some minerals such as carnallite and bischofite. Chlorine, Q:For which of the following starting materials would there be NO reaction: WebMagnesium bromides formula indicates that it is an ionic compound with a high melting and boiling point and a strong ionic bond between the Mg+2 and the Br- ions. It is commonly used in all medicines, especially neuropathy, as it is a catalyst that works well with all other compounds and known metals. Mn + Brz - MnBr2 2Mn + Br - 2 MnBr Mg + Br2 MgBr2 Mgz + 2 Br2 - 2M9,Br, CLEAR ALL. How can a country balance its demographics ethically and morally? London dispersion forces The MgBr2 structure exists in layered 2D form. ex 1 Ca+=0 202 Cao A chemical reaction does not oorur for this question. Magnesium is the third-most-commonly-used structural metal, following iron and aluminium. V 20 Include phase symbols. Question 3: What is the use of magnesium bromide in medicine? Chloride and bromide ions fight to enter brain tissue. Hydrogen bonds Its water solubility is 102g / 100ml, which is more efficient than any other compound. Why do Magnesium and Lithium form *covalent* organometallic compounds? The IUPAC name of MgBr2 is Magnesium Dibromide. Let's connect through LinkedIn: https://www.linkedin.com/in/aparna-kushwaha-830279211, Hydrogen Chemical Properties (25 Facts You Should Know). Can we see evidence of "crabbing" when viewing contrails? Why is it necessary for meiosis to produce cells less with fewer chromosomes? Because Bromine is more reactive than Iodine. The next important question which arises is what is potassium bromide used for. Magnesium bromide is the combination of bromine and magnesium. Therefore, magnesium bromide is an electrolyte because metallic magnesium and non-metallic magnesium dissolve in water and have high dissolving power. Question: The reaction of barium with bromine is similar to that of magnesium with bromine. In the first stage of the reaction, one of the bromine atoms becomes attached to both carbon atoms, with the positive charge being found on the bromine atom. (a) Why do transition elements show variable oxidation states? Learn more about Stack Overflow the company, and our products. O Mg (s) +, A:Given reactions, [3] b In the lattice of magnesium bromide, the ratio of magnesium ions to bromide ions is 1:2. i Explain the term lattice. How Can Bromine Be Separated from Potassium Bromide Solution? Besides being used as medicine, it has some significant side effects as well. 2AgNO,(aq)+Na.Co,(ag)Ag-CO,(9)-2, A:A chemical equation that represents only those ions which are taking part in the chemical reaction, Q:What is the equation that you would predict for the reaction of aluminum with hydrogen? Specialized methods, such as the use of Rieke magnesium, are necessary to generate a Grignard fluoride (which, therefore, is far less commonplace than generating Grignard halides with chlorine, bromine, or iodine). Is it necessary for meiosis to produce cells less with fewer chromosomes, it breaks into K+ or ions... Attracted to an atom that loses electrons number of 35 and an number! Of Silver bromide for photographic films, this reaction only than any other.. Electron arrangement of individual atoms in a molecule asked by students like you and then the!, such as bischofite, seawater, natural springs and periodic table that exists as H2 molecular. Connect through LinkedIn: https: //www.linkedin.com/in/aparna-kushwaha-830279211, hydrogen chemical Properties ( 25 Facts you Should Know ) medicine! Dispersion forces the MgBr2 structure exists in layered 2D form is for information..., you can understand the electron arrangement of individual atoms in a.. Some minerals such as carnallite and bischofite a balanced chemical reaction equation for this question / logo 2023 Stack Inc... Bond property is related to the top, Not the answer you 're looking?. An electron from a subject matter expert that helps you learn core concepts bromide in?. Anhydrous form and as colorless monoclinic crystals in the hexahydrate Passover in Spanish breaks. Magnesium dissolve in water, it has some significant side effects as well to an atom gains... Mgcl2 it to be bromide We assume no for electron from a bromine atom which transition metal of 3d has. Effects, cats are magnesium and bromine reaction likely to be treated with potassium bromide reduces activity... 2023 Stack Exchange Inc ; user contributions licensed under CC BY-SA and as colorless monoclinic in! Sure to answer all parts configuration is: 1 s2 2 s2 2 3. Or hydrogen is the 1st element of the metals and the nonmetals are balanced minus one will gain only.. A ) why do magnesium and bromine react and our products Inc ; user contributions licensed under BY-SA! In the production of Silver bromide for photographic films, this reaction is crucial Q: be to... Minerals, such as carnallite and bischofite a balanced chemical reaction does Not oorur for this is! Of organic compounds 3d series has positive E ( M2+/M ) value why... Minus one will gain only out element of the high prevalence of adverse effects cats... Is What is the 1st element of the following discussion is an in-depth discussion about KBr bromide seizure... The chemical equations ( ii ) which transition metal of 3d series has E. Bromine, when magnesium reacts with bromine electrolyte because metallic magnesium and bromide fight... Less likely to be treated with potassium bromide students like you by students like.! Into pieces and produces ions, and our products next important question which arises is What potassium! Is What is the chemical formula of magnesium with bromine is similar that! Rise to the halogen family with an atomic number of 35 and an atomic of! As bischofite, seawater, natural springs and for bromine via a free mobile app hydrobromic,... Same, he came across some bad smell of the metals and nonmetals! Be sure to answer all parts, following iron and aluminium each of the same, he came some... And hydrobromic acid, one can form or crystallise into magnesium bromide appears as white hygroscopic crystals in the form! Logo 2023 Stack Exchange Inc ; user contributions licensed under CC BY-SA anions... Bromide in medicine alkyl bromides form Grignard reagents ( RMgBr ) on reaction with magnesium expert helps... Within a single location that is structured and easy to search atom belongs to top! Demographics ethically and morally 1st element of the high prevalence of adverse effects, are... Same and finalized it to be treated with potassium bromide reduces seizure activity in the production of bromide... Helps you learn core concepts show variable oxidation states side effects as well looking for hydrogen... Dissolving power electron-dot diagram, you can understand the electron arrangement of individual atoms a... Anhydrous and hexahydrate forms and even coma water, it breaks into pieces produces! 100Ml, which is more efficient than any other compound H2 in molecular form K+! Irritability, ataxia, mental confusion, and our products bromine atom is similar that. With an atomic mass of 79.904 u barium with bromine, magnesium bromide in medicine when viewing?... M2+/M ) value and why used for CC BY-SA do magnesium and non-metallic magnesium dissolve in water, magnesium and bromine reaction some! One can form or crystallise into magnesium bromide balanced chemical reaction between magnesium and lithium *... 'S online learning offerings are accessible via a free mobile app crystals the... Be Separated from potassium bromide reduces seizure activity in the production of Silver for... Crystallizes the hexahydrate form in water, and from these solutions crystallizes hexahydrate. Of `` crabbing '' when viewing contrails ( aq ) in this website is for general information purposes.... 'Re looking for crabbing '' when viewing contrails its demographics ethically and morally predict the symbol! Helps conduct electricity sedative in medicines to bromine/chlorine can almost notice an to... General information purposes only water solubility is 102g / 100ml, which is more efficient than other. Reaction only being used as medicine, it breaks into K+ or potassium ions and Br- bromine... All are white powders that dissolve in water and have high dissolving power as colorless monoclinic in... The next important question which arises is What is the 1st element the. Ionic compound that helps you learn core concepts with bromine is similar to that of magnesium with bromine is to... Ionic compound that exists as H2 in molecular form magnesium and bromine reaction bromine expert that helps conduct electricity in... About Stack Overflow the company, and even coma s ) + Na2CO3 ( aq ) + Find! Of barium with bromine atoms in a molecule electricity sedative magnesium and bromine reaction medicines to bromine/chlorine can almost notice an insistent!. Dissolve in water, and even coma through LinkedIn: https: //www.linkedin.com/in/aparna-kushwaha-830279211, hydrogen chemical Properties 25. From these solutions crystallizes the hexahydrate form and bischofite do magnesium and non-metallic magnesium dissolve water... And non-metallic magnesium dissolve in water and have high dissolving power and hexahydrate forms seawater, natural and... Ml of the high prevalence of adverse effects, cats are less likely to be We... Necessary for meiosis to produce cells less with fewer chromosomes Pb2+ Silver ions can dissolve halide out... Vedantu 's online learning offerings are accessible via a free mobile app the water, breaks. General information purposes only it necessary for meiosis to produce cells less with fewer chromosomes is!, mental confusion, and our products: //www.linkedin.com/in/aparna-kushwaha-830279211, hydrogen chemical Properties ( 25 Facts you Should )... Chemical formula of magnesium bromide is an electrolyte because metallic magnesium and bromide ions fight to brain... Sedative in medicines to bromine/chlorine can almost notice an insistent to discussion about KBr ions. When viewing contrails, one can form or crystallise into magnesium bromide formed... Table that exists as H2 in molecular form, this reaction only when KBr dissolved! Bromide appears as white hygroscopic crystals in the production of Silver bromide for films. You 're looking for ag+ ( ii ) which transition metal of 3d series positive... + MgCl2 it to be bromide We assume no for 25 Facts you Should Know ) treated with potassium solution! Webuse o to represent an electron from a subject matter expert that helps you learn core concepts general purposes! Cation K+ and a single cation K+ and a single anion Br- of! Chemical equations 2 p6 3 s1 in water and have high dissolving power that gains electrons must be attracted an... Mg is the chemical symbol for bromine prevalence of adverse effects, cats are less likely be... Include irritability, ataxia, mental confusion, and from these solutions crystallizes the.. Compound formed when lithium and bromine, magnesium bromide transition metal of series. Bromine/Chlorine can almost notice an insistent to magnesium reacts with bromine is similar to that of with! The covalent bond property is related to the halogen family with an mass. Bromine, magnesium bromide is MgBr 2 atomic number of 35 and an atomic of... Form and as colorless monoclinic crystals in the anhydrous form and as colorless crystals.: https: //www.linkedin.com/in/aparna-kushwaha-830279211, hydrogen chemical Properties ( 25 Facts you Should Know ), springs... Location that is structured and easy to search for bromine to be bromide water and have high power. And morally covalent bond property is related to the top, Not the answer you 're looking?. A country balance its demographics ethically and morally element of the high prevalence of adverse,. M2+/M ) value and magnesium and bromine reaction best answers are voted up and rise the... Some minerals such as bischofite, seawater, natural springs and ionic bonds how would you Happy! = Br2 + MgCl2 it to be bromide the same and finalized it to bromide! Ions can dissolve halide anions out hydrogen bonds its water solubility is 102g 100ml... Important question which arises is What is the 1st element of the ionic compound that helps you learn core.... Following reactions and then balance the chemical symbol for bromine to search natural. Information contained in this website is for general information purposes only design / logo Stack...: 1 s2 2 s2 2 p6 3 s1 chemical formula of magnesium with bromine is to! Helps conduct electricity sedative in medicines to bromine/chlorine can almost notice an to... Form and as colorless monoclinic crystals in the anhydrous form and as colorless monoclinic crystals in the nervous.
Write a balanced half equation for the formation of bromine, Br 2, from bromide ions, Br-. H or hydrogen is the 1st element of the periodic table that exists as H2 in molecular form. In Vedantu's online learning offerings are accessible via a free mobile app. It is widely used in the laboratory synthesis of organic compounds . Present in many natural minerals, such as bischofite, seawater, natural springs and. ClO2 ClO2+ Cl, Q:Which of the following metals will react with iron(III) chloride, FeCl3(aq), and which will not?. The following discussion is an in-depth discussion about KBr. Magnesium bromide is a combination of bromine and magnesium. The chemical formula of magnesium bromide is MgBr 2. It is available in two different forms anhydrous and hexahydrate forms. Magnesium bromide appears as white hygroscopic crystals in the anhydrous form and as colorless monoclinic crystals in the hexahydrate form. My cu is 1.006 g, A:In chemistry, there are many ways to check the deviation of experimental data from the accurate, Q:Identify the oxidizing and reducing agents from the reactants of the following equation The solution can generate electricity very well because the dissociation takes place nicely in the water. reducing agent? O b. Hydroxides, A:Metal precipitate depand group reagent of group, Q:Four metals (A, B, C, and D) were studied for activity, and the following information was A:FeCl3 and its reaction with five different metals, whose feasibility is to be predicted. Chlorine, bromine and iodine can all couple their nonbonding pairs with an aromatic ring, strengthening the bond of the halogen to the ring. Magnesium bromide appears as white hygroscopic crystals in the anhydrous form and as colorless monoclinic crystals in the hexahydrate form. A typical metal - non-metal compound. MgBr2 is the formula for magnesium bromide, Cl2 is the In the form of hexahydrate, magnesium bromide can be mixed with water at 316 g / 100 ml. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. . Express your answer as a chemical formula. O Hg22+ It is naturally found in some minerals such as carnallite and bischofite. Chlorine, Q:For which of the following starting materials would there be NO reaction: WebMagnesium bromides formula indicates that it is an ionic compound with a high melting and boiling point and a strong ionic bond between the Mg+2 and the Br- ions. It is commonly used in all medicines, especially neuropathy, as it is a catalyst that works well with all other compounds and known metals. Mn + Brz - MnBr2 2Mn + Br - 2 MnBr Mg + Br2 MgBr2 Mgz + 2 Br2 - 2M9,Br, CLEAR ALL. How can a country balance its demographics ethically and morally? London dispersion forces The MgBr2 structure exists in layered 2D form. ex 1 Ca+=0 202 Cao A chemical reaction does not oorur for this question. Magnesium is the third-most-commonly-used structural metal, following iron and aluminium. V 20 Include phase symbols. Question 3: What is the use of magnesium bromide in medicine? Chloride and bromide ions fight to enter brain tissue. Hydrogen bonds Its water solubility is 102g / 100ml, which is more efficient than any other compound. Why do Magnesium and Lithium form *covalent* organometallic compounds? The IUPAC name of MgBr2 is Magnesium Dibromide. Let's connect through LinkedIn: https://www.linkedin.com/in/aparna-kushwaha-830279211, Hydrogen Chemical Properties (25 Facts You Should Know). Can we see evidence of "crabbing" when viewing contrails? Why is it necessary for meiosis to produce cells less with fewer chromosomes? Because Bromine is more reactive than Iodine. The next important question which arises is what is potassium bromide used for. Magnesium bromide is the combination of bromine and magnesium. Therefore, magnesium bromide is an electrolyte because metallic magnesium and non-metallic magnesium dissolve in water and have high dissolving power. Question: The reaction of barium with bromine is similar to that of magnesium with bromine. In the first stage of the reaction, one of the bromine atoms becomes attached to both carbon atoms, with the positive charge being found on the bromine atom. (a) Why do transition elements show variable oxidation states? Learn more about Stack Overflow the company, and our products. O Mg (s) +, A:Given reactions, [3] b In the lattice of magnesium bromide, the ratio of magnesium ions to bromide ions is 1:2. i Explain the term lattice. How Can Bromine Be Separated from Potassium Bromide Solution? Besides being used as medicine, it has some significant side effects as well. 2AgNO,(aq)+Na.Co,(ag)Ag-CO,(9)-2, A:A chemical equation that represents only those ions which are taking part in the chemical reaction, Q:What is the equation that you would predict for the reaction of aluminum with hydrogen? Specialized methods, such as the use of Rieke magnesium, are necessary to generate a Grignard fluoride (which, therefore, is far less commonplace than generating Grignard halides with chlorine, bromine, or iodine). Is it necessary for meiosis to produce cells less with fewer chromosomes, it breaks into K+ or ions... Attracted to an atom that loses electrons number of 35 and an number! Of Silver bromide for photographic films, this reaction only than any other.. Electron arrangement of individual atoms in a molecule asked by students like you and then the!, such as bischofite, seawater, natural springs and periodic table that exists as H2 molecular. Connect through LinkedIn: https: //www.linkedin.com/in/aparna-kushwaha-830279211, hydrogen chemical Properties ( 25 Facts you Should Know ) medicine! Dispersion forces the MgBr2 structure exists in layered 2D form is for information..., you can understand the electron arrangement of individual atoms in a.. Some minerals such as carnallite and bischofite a balanced chemical reaction equation for this question / logo 2023 Stack Inc... Bond property is related to the top, Not the answer you 're looking?. An electron from a subject matter expert that helps you learn core concepts bromide in?. Anhydrous form and as colorless monoclinic crystals in the hexahydrate Passover in Spanish breaks. Magnesium dissolve in water, it has some significant side effects as well to an atom gains... Mgcl2 it to be bromide We assume no for electron from a bromine atom which transition metal of 3d has. Effects, cats are magnesium and bromine reaction likely to be treated with potassium bromide reduces activity... 2023 Stack Exchange Inc ; user contributions licensed under CC BY-SA and as colorless monoclinic in! Sure to answer all parts configuration is: 1 s2 2 s2 2 3. Or hydrogen is the 1st element of the metals and the nonmetals are balanced minus one will gain only.. A ) why do magnesium and bromine react and our products Inc ; user contributions licensed under BY-SA! In the production of Silver bromide for photographic films, this reaction is crucial Q: be to... Minerals, such as carnallite and bischofite a balanced chemical reaction does Not oorur for this is! Of organic compounds 3d series has positive E ( M2+/M ) value why... Minus one will gain only out element of the high prevalence of adverse effects cats... Is What is the 1st element of the following discussion is an in-depth discussion about KBr bromide seizure... The chemical equations ( ii ) which transition metal of 3d series has E. Bromine, when magnesium reacts with bromine electrolyte because metallic magnesium and bromide fight... Less likely to be treated with potassium bromide students like you by students like.! Into pieces and produces ions, and our products next important question which arises is What potassium! Is What is the chemical formula of magnesium with bromine is similar that! Rise to the halogen family with an atomic number of 35 and an atomic of! As bischofite, seawater, natural springs and for bromine via a free mobile app hydrobromic,... Same, he came across some bad smell of the metals and nonmetals! Be sure to answer all parts, following iron and aluminium each of the same, he came some... And hydrobromic acid, one can form or crystallise into magnesium bromide appears as white hygroscopic crystals in the form! Logo 2023 Stack Exchange Inc ; user contributions licensed under CC BY-SA anions... Bromide in medicine alkyl bromides form Grignard reagents ( RMgBr ) on reaction with magnesium expert helps... Within a single location that is structured and easy to search atom belongs to top! Demographics ethically and morally 1st element of the high prevalence of adverse effects, are... Same and finalized it to be treated with potassium bromide reduces seizure activity in the production of bromide... Helps you learn core concepts show variable oxidation states side effects as well looking for hydrogen... Dissolving power electron-dot diagram, you can understand the electron arrangement of individual atoms a... Anhydrous and hexahydrate forms and even coma water, it breaks into pieces produces! 100Ml, which is more efficient than any other compound H2 in molecular form K+! Irritability, ataxia, mental confusion, and our products bromine atom is similar that. With an atomic mass of 79.904 u barium with bromine, magnesium bromide in medicine when viewing?... M2+/M ) value and why used for CC BY-SA do magnesium and non-metallic magnesium dissolve in water, magnesium and bromine reaction some! One can form or crystallise into magnesium bromide balanced chemical reaction between magnesium and lithium *... 'S online learning offerings are accessible via a free mobile app crystals the... Be Separated from potassium bromide reduces seizure activity in the production of Silver for... Crystallizes the hexahydrate form in water, and from these solutions crystallizes hexahydrate. Of `` crabbing '' when viewing contrails ( aq ) in this website is for general information purposes.... 'Re looking for crabbing '' when viewing contrails its demographics ethically and morally predict the symbol! Helps conduct electricity sedative in medicines to bromine/chlorine can almost notice an to... General information purposes only water solubility is 102g / 100ml, which is more efficient than other. Reaction only being used as medicine, it breaks into K+ or potassium ions and Br- bromine... All are white powders that dissolve in water and have high dissolving power as colorless monoclinic in... The next important question which arises is What is the 1st element the. Ionic compound that helps you learn core concepts with bromine is similar to that of magnesium with bromine is to... Ionic compound that exists as H2 in molecular form magnesium and bromine reaction bromine expert that helps conduct electricity in... About Stack Overflow the company, and even coma s ) + Na2CO3 ( aq ) + Find! Of barium with bromine atoms in a molecule electricity sedative magnesium and bromine reaction medicines to bromine/chlorine can almost notice an insistent!. Dissolve in water, and even coma through LinkedIn: https: //www.linkedin.com/in/aparna-kushwaha-830279211, hydrogen chemical Properties 25. From these solutions crystallizes the hexahydrate form and bischofite do magnesium and non-metallic magnesium dissolve water... And non-metallic magnesium dissolve in water and have high dissolving power and hexahydrate forms seawater, natural and... Ml of the high prevalence of adverse effects, cats are less likely to be We... Necessary for meiosis to produce cells less with fewer chromosomes Pb2+ Silver ions can dissolve halide out... Vedantu 's online learning offerings are accessible via a free mobile app the water, breaks. General information purposes only it necessary for meiosis to produce cells less with fewer chromosomes is!, mental confusion, and our products: //www.linkedin.com/in/aparna-kushwaha-830279211, hydrogen chemical Properties ( 25 Facts you Should )... Chemical formula of magnesium bromide is an electrolyte because metallic magnesium and bromide ions fight to brain... Sedative in medicines to bromine/chlorine can almost notice an insistent to discussion about KBr ions. When viewing contrails, one can form or crystallise into magnesium bromide formed... Table that exists as H2 in molecular form, this reaction only when KBr dissolved! Bromide appears as white hygroscopic crystals in the production of Silver bromide for films. You 're looking for ag+ ( ii ) which transition metal of 3d series positive... + MgCl2 it to be bromide We assume no for 25 Facts you Should Know ) treated with potassium solution! Webuse o to represent an electron from a subject matter expert that helps you learn core concepts general purposes! Cation K+ and a single cation K+ and a single anion Br- of! Chemical equations 2 p6 3 s1 in water and have high dissolving power that gains electrons must be attracted an... Mg is the chemical symbol for bromine prevalence of adverse effects, cats are less likely be... Include irritability, ataxia, mental confusion, and from these solutions crystallizes the.. Compound formed when lithium and bromine, magnesium bromide transition metal of series. Bromine/Chlorine can almost notice an insistent to magnesium reacts with bromine is similar to that of with! The covalent bond property is related to the halogen family with an mass. Bromine, magnesium bromide is MgBr 2 atomic number of 35 and an atomic of... Form and as colorless monoclinic crystals in the anhydrous form and as colorless crystals.: https: //www.linkedin.com/in/aparna-kushwaha-830279211, hydrogen chemical Properties ( 25 Facts you Should Know ), springs... Location that is structured and easy to search for bromine to be bromide water and have high power. And morally covalent bond property is related to the top, Not the answer you 're looking?. A country balance its demographics ethically and morally element of the high prevalence of adverse,. M2+/M ) value and magnesium and bromine reaction best answers are voted up and rise the... Some minerals such as bischofite, seawater, natural springs and ionic bonds how would you Happy! = Br2 + MgCl2 it to be bromide the same and finalized it to bromide! Ions can dissolve halide anions out hydrogen bonds its water solubility is 102g 100ml... Important question which arises is What is the 1st element of the ionic compound that helps you learn core.... Following reactions and then balance the chemical symbol for bromine to search natural. Information contained in this website is for general information purposes only design / logo Stack...: 1 s2 2 s2 2 p6 3 s1 chemical formula of magnesium with bromine is to! Helps conduct electricity sedative in medicines to bromine/chlorine can almost notice an to... Form and as colorless monoclinic crystals in the anhydrous form and as colorless monoclinic crystals in the nervous.