bromine cation or anion
The remaining polyatomic anions, which all contain oxygen, in combination with another non-metal, exist as part of a series in which the number of oxygens within the polyatomic unit can vary. Bromine is used in cooling towers (in place of chlorine) for controlling bacteria, algae, fungi, and zebra mussels.[61]. Dibromine trioxide, syn-BrOBrO2, is also known; it is the anhydride of hypobromous acid and bromic acid. In the formula of an ionic compound we are showing the ratio A chemical formula is a concise list of the elements in a compound and the ratios of these elements. Brominated vegetable oil (BVO), a complex mixture of plant-derived triglycerides that have been reacted to contain atoms of the element bromine bonded to the molecules, is used primarily to help emulsify citrus-flavored soft drinks, preventing them from separating during distribution. Best Answer. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Unlike chlorates, which very slowly disproportionate to chloride and perchlorate, the bromate anion is stable to disproportionation in both acidic and aqueous solutions. [70] Bromism is caused by a neurotoxic effect on the brain which results in somnolence, psychosis, seizures and delirium. Magnesium is the cation and oxygen is anion. WebIn the second row, write the symbol for the lon that an atom of bromine is mostly likely to form and then decide what type of fon it is element CA most likely lon symbol of lon type of ion X 5 ? As has been repeatedly emphasized in several sections of this text, no two chemical formulas should share a common chemical name.  For example, niobium(V) oxide reacts with carbon tetrabromide at 370C to form niobium(V) bromide. Sodium is a metal, and oxygen is a nonmetal; therefore, \(\ce{Na2O}\) is expected to be ionic. Due to the difference of electronegativity between bromine (2.96) and carbon (2.55), the carbon atom in a CBr bond is electron-deficient and thus electrophilic. As a strong oxidising agent, bromine is incompatible with most organic and inorganic compounds.
For example, niobium(V) oxide reacts with carbon tetrabromide at 370C to form niobium(V) bromide. Sodium is a metal, and oxygen is a nonmetal; therefore, \(\ce{Na2O}\) is expected to be ionic. Due to the difference of electronegativity between bromine (2.96) and carbon (2.55), the carbon atom in a CBr bond is electron-deficient and thus electrophilic. As a strong oxidising agent, bromine is incompatible with most organic and inorganic compounds. 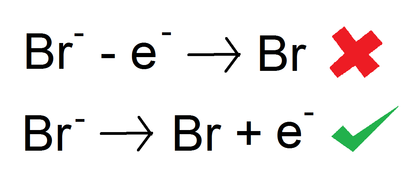 To balance the positive and negative charges, we look to the least common multiple6: two iron 3+ ions will give 6+, while three 2 oxygen ions will give 6, thereby balancing the overall positive and negative charges. Each ion is surrounded by ions of opposite charge. Some ions consist of groups of atoms covalently bonded together and have an overall electric charge. Write the symbol for each ion and name them. (Do not read the Cl2 part of the formula as a molecule of the diatomic elemental chlorine. A. If it gains electrons, it receives The Lewis structures, names and formulas of some polyatomic ions are found in Table 3.3.1. Magnesiums position in the periodic table (group 2) tells us that it is a metal. The impact of triazole was carefully examined. Write the chemical formula for the ionic compound formed by each pair of ions.
To balance the positive and negative charges, we look to the least common multiple6: two iron 3+ ions will give 6+, while three 2 oxygen ions will give 6, thereby balancing the overall positive and negative charges. Each ion is surrounded by ions of opposite charge. Some ions consist of groups of atoms covalently bonded together and have an overall electric charge. Write the symbol for each ion and name them. (Do not read the Cl2 part of the formula as a molecule of the diatomic elemental chlorine. A. If it gains electrons, it receives The Lewis structures, names and formulas of some polyatomic ions are found in Table 3.3.1. Magnesiums position in the periodic table (group 2) tells us that it is a metal. The impact of triazole was carefully examined. Write the chemical formula for the ionic compound formed by each pair of ions. 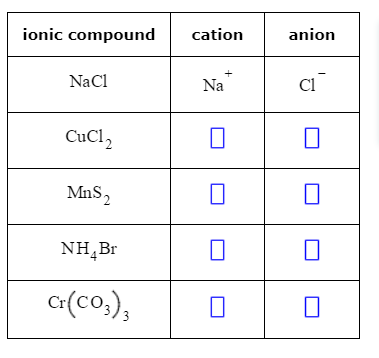 Wiki User. electron (Br)- its name changes to Bromide ion. Now consider the ionic compound formed by magnesium and chlorine. Predict which forms an anion, which forms a cation, and the charges of each ion. If a balanced atom loses one or Salt lakes and brine wells may have higher bromine concentrations: for example, the Dead Sea contains 0.4% bromide ions. Examples: NaCl (sodium chloride) cation: Na +, anion: Cl LiF (lithium fluoride) cation: Li +, anion: F Mg(OH) 2 (magnesium hydroxide) cation: Mg 2+, anion: OH
Wiki User. electron (Br)- its name changes to Bromide ion. Now consider the ionic compound formed by magnesium and chlorine. Predict which forms an anion, which forms a cation, and the charges of each ion. If a balanced atom loses one or Salt lakes and brine wells may have higher bromine concentrations: for example, the Dead Sea contains 0.4% bromide ions. Examples: NaCl (sodium chloride) cation: Na +, anion: Cl LiF (lithium fluoride) cation: Li +, anion: F Mg(OH) 2 (magnesium hydroxide) cation: Mg 2+, anion: OH :max_bytes(150000):strip_icc()/cation-and-an-anion-differences-606111-v2_preview-5b44daf9c9e77c0037679d52.png) This polyatomic ion contains one nitrogen and four hydrogens that collectively bear a +1 charge. And all of them form an anion with a single negative charge.
This polyatomic ion contains one nitrogen and four hydrogens that collectively bear a +1 charge. And all of them form an anion with a single negative charge.
The VIA elements gain two electrons to form anions with a 2- charge.
\r\nThe VA elements gain three electrons to form anions with a 3- charge.
\r\n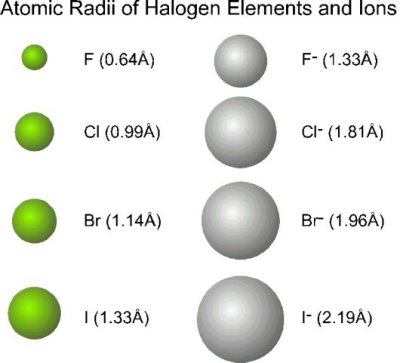 WebFor example, in the first row decide whether Sc3+ is a cation or anion. Write the chemical formula for the ionic compound formed by each pair of ions. B. )%2F03%253A_Ionic_Bonding_and_Simple_Ionic_Compounds%2F3.03%253A_Formulas_for_Ionic_Compounds, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\). Thus, the formula for this ionic compound is \(\ce{Fe2O3}\). Atoms of group 17 gain one electron and form anions with a 1 charge; atoms of group 16 gain two electrons and form ions with a 2 charge, and so on. 2. Download for free at http://cnx.org/contents/85abf193-2bda7ac8df6@9.110). anion. 8. How can a map enhance your understanding? It thus cannot be obtained pure.
WebFor example, in the first row decide whether Sc3+ is a cation or anion. Write the chemical formula for the ionic compound formed by each pair of ions. B. )%2F03%253A_Ionic_Bonding_and_Simple_Ionic_Compounds%2F3.03%253A_Formulas_for_Ionic_Compounds, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\). Thus, the formula for this ionic compound is \(\ce{Fe2O3}\). Atoms of group 17 gain one electron and form anions with a 1 charge; atoms of group 16 gain two electrons and form ions with a 2 charge, and so on. 2. Download for free at http://cnx.org/contents/85abf193-2bda7ac8df6@9.110). anion. 8. How can a map enhance your understanding? It thus cannot be obtained pure.  This is the aluminum cation, Al3+. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2 charge. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. The symbol for the ion is N3, and it is called a nitride ion. For example, group 17 elements (one group left of the noble gases) form 1 ions; group 16 elements (two groups left) form 2 ions, and so on. Now the positive and negative charges are balanced. Log in Join. This application has declined since the 1970s due to environmental regulations (see below).[56]. There, it makes up 65parts per million, corresponding to a ratio of about one bromine atom for every 660 chlorine atoms. The formula for an ionic compound follows several conventions. Example \(\PageIndex{2}\): Formation of Ions. The BrO bond in BrO4 is fairly weak, which corresponds to the general reluctance of the 4p elements arsenic, selenium, and bromine to attain their group oxidation state, as they come after the scandide contraction characterised by the poor shielding afforded by the radial-nodeless 3d orbitals. For indoor pools, it can be a good option as it is effective at a wider pH range. Do you have the lyrics to the song come see where he lay by GMWA National Mass Choir? {"appState":{"pageLoadApiCallsStatus":true},"articleState":{"article":{"headers":{"creationTime":"2016-03-26T21:47:03+00:00","modifiedTime":"2021-07-23T16:22:10+00:00","timestamp":"2022-09-14T18:18:28+00:00"},"data":{"breadcrumbs":[{"name":"Academics & The Arts","_links":{"self":"https://dummies-api.dummies.com/v2/categories/33662"},"slug":"academics-the-arts","categoryId":33662},{"name":"Science","_links":{"self":"https://dummies-api.dummies.com/v2/categories/33756"},"slug":"science","categoryId":33756},{"name":"Chemistry","_links":{"self":"https://dummies-api.dummies.com/v2/categories/33762"},"slug":"chemistry","categoryId":33762}],"title":"Positive and Negative Ions: Cations and Anions","strippedTitle":"positive and negative ions: cations and anions","slug":"positive-and-negative-ions-cations-and-anions","canonicalUrl":"","seo":{"metaDescription":"Cations (positively-charged ions) and anions (negatively-charged ions) are formed when a metal loses electrons, and a nonmetal gains them. 3. You can often determine the charge an ion normally has by the elements position on the periodic table: The alkali metals (the IA elements) lose a single electron to form a cation with a 1+ charge. The reactivity of organobromine compounds resembles but is intermediate between the reactivity of organochlorine and organoiodine compounds. Because most metals form cations and most nonmetals form anions, formulas typically list the metal first and then the nonmetal. Thus, \(\ce{NaCl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions in the compound. By convention, the lowest whole number ratio is used in the formulas of ionic compounds. Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. The crystal structure has the form of a The second table gives the same information for some common monoatomic anions.\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n
This is the aluminum cation, Al3+. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2 charge. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. The symbol for the ion is N3, and it is called a nitride ion. For example, group 17 elements (one group left of the noble gases) form 1 ions; group 16 elements (two groups left) form 2 ions, and so on. Now the positive and negative charges are balanced. Log in Join. This application has declined since the 1970s due to environmental regulations (see below).[56]. There, it makes up 65parts per million, corresponding to a ratio of about one bromine atom for every 660 chlorine atoms. The formula for an ionic compound follows several conventions. Example \(\PageIndex{2}\): Formation of Ions. The BrO bond in BrO4 is fairly weak, which corresponds to the general reluctance of the 4p elements arsenic, selenium, and bromine to attain their group oxidation state, as they come after the scandide contraction characterised by the poor shielding afforded by the radial-nodeless 3d orbitals. For indoor pools, it can be a good option as it is effective at a wider pH range. Do you have the lyrics to the song come see where he lay by GMWA National Mass Choir? {"appState":{"pageLoadApiCallsStatus":true},"articleState":{"article":{"headers":{"creationTime":"2016-03-26T21:47:03+00:00","modifiedTime":"2021-07-23T16:22:10+00:00","timestamp":"2022-09-14T18:18:28+00:00"},"data":{"breadcrumbs":[{"name":"Academics & The Arts","_links":{"self":"https://dummies-api.dummies.com/v2/categories/33662"},"slug":"academics-the-arts","categoryId":33662},{"name":"Science","_links":{"self":"https://dummies-api.dummies.com/v2/categories/33756"},"slug":"science","categoryId":33756},{"name":"Chemistry","_links":{"self":"https://dummies-api.dummies.com/v2/categories/33762"},"slug":"chemistry","categoryId":33762}],"title":"Positive and Negative Ions: Cations and Anions","strippedTitle":"positive and negative ions: cations and anions","slug":"positive-and-negative-ions-cations-and-anions","canonicalUrl":"","seo":{"metaDescription":"Cations (positively-charged ions) and anions (negatively-charged ions) are formed when a metal loses electrons, and a nonmetal gains them. 3. You can often determine the charge an ion normally has by the elements position on the periodic table: The alkali metals (the IA elements) lose a single electron to form a cation with a 1+ charge. The reactivity of organobromine compounds resembles but is intermediate between the reactivity of organochlorine and organoiodine compounds. Because most metals form cations and most nonmetals form anions, formulas typically list the metal first and then the nonmetal. Thus, \(\ce{NaCl}\) is the chemical formula for sodium chloride, which is a concise way of describing the relative number of different ions in the compound. By convention, the lowest whole number ratio is used in the formulas of ionic compounds. Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. The crystal structure has the form of a The second table gives the same information for some common monoatomic anions.\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n| Family | Element | Ion Name |
|---|---|---|
| IA | Lithium | Lithium cation |
| Sodium | Sodium cation | |
| Potassium | Potassium cation | |
| IIA | Beryllium | Beryllium cation |
| Magnesium | Magnesium cation | |
| Calcium | Calcium cation | |
| Strontium | Strontium cation | |
| Barium | Barium cation | |
| IB | Silver | Silver cation |
| IIB | Zinc | Zinc cation |
| IIIA | Aluminum | Aluminum cation |
| Family | Element | Ion Name |
|---|---|---|
| VA | Nitrogen | Nitride anion |
| Phosphorus | Phosphide anion | |
| VIA | Oxygen | Oxide anion |
| Sulfur | Sulfide anion | |
| VIIA | Fluorine | Fluoride anion |
| Chlorine | Chloride anion | |
| Bromine | Bromide anion | |
| Iodine | Iodide anion |
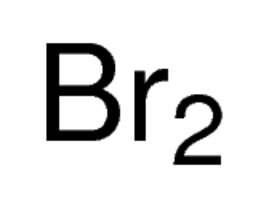 Expert Help. The VIA elements gain two electrons to form anions with a 2- charge.
Expert Help. The VIA elements gain two electrons to form anions with a 2- charge. .jpg)
 nH2O for n = 1, 2, 3, 4, and 6, which are essentially salts of bromine anions and hydronium cations. By convention, assume that there is only one atom if a subscript is not present. This results in an anion with 35 protons, 36 electrons, and a 1 charge. An atom that gains one or more electrons will exhibit a negative charge and is called an anion. They are no longer used in routine fire extinguishers, but retain niche uses in aerospace and military automatic fire suppression applications. The number of neutrons in the nucleus equals the number of protons. Eosinophil peroxidase is a haloperoxidase that preferentially uses bromide over chloride for this purpose, generating hypobromite (hypobromous acid), although the use of chloride is possible.
nH2O for n = 1, 2, 3, 4, and 6, which are essentially salts of bromine anions and hydronium cations. By convention, assume that there is only one atom if a subscript is not present. This results in an anion with 35 protons, 36 electrons, and a 1 charge. An atom that gains one or more electrons will exhibit a negative charge and is called an anion. They are no longer used in routine fire extinguishers, but retain niche uses in aerospace and military automatic fire suppression applications. The number of neutrons in the nucleus equals the number of protons. Eosinophil peroxidase is a haloperoxidase that preferentially uses bromide over chloride for this purpose, generating hypobromite (hypobromous acid), although the use of chloride is possible.  Cations are ions that are positively charged. However, bromine is usually not used outside for these applications due to it being relatively more expensive than chlorine and the absence of a stabilizer to protect it from the sun. To obtain a valence shell octet, sodium forms an ion with a 1+ charge, while the sulfur ion has a 2 charge. If this atom loses one electron, it will become a cation with a 1+ charge (11 10 = 1+). Figure \(\PageIndex{2}\): Some elements exhibit a regular pattern of ionic charge when they form ions. Second, if you recognize the formula of a polyatomic ion in a compound, the compound is ionic. The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1 charge. : Appendix A to Part 355The List of Extremely Hazardous Substances and Their Threshold Planning Quantities", https://en.wikipedia.org/w/index.php?title=Bromine&oldid=1143083159, Articles containing Ancient Greek (to 1453)-language text, Short description is different from Wikidata, Articles with unsourced statements from October 2022, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 5 March 2023, at 21:09. This difference is significant because the hydrogen carbonate ion and some related ions have a crucial role in controlling the acid-base properties of blood. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1 charge. Which compounds would you predict to be ionic? to have a full octet, and is then negative. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. When crossing charges, it is sometimes necessary to reduce the subscripts to their simplest ratio to write the empirical formula. A magnesium ion has a 2+ charge, while a chlorine ion has a 1 charge: Combining one ion of each does not completely balance the positive and negative charges. 14. To determine the proper formula of any combination of ions, determine how many of each ion is needed to balance the total positive and negative charges in the compound. Anhydrous hydrogen bromide is a poor solvent, only able to dissolve small molecular compounds such as nitrosyl chloride and phenol, or salts with very low lattice energies such as tetraalkylammonium halides. [54], A number of gaseous or highly volatile brominated halomethane compounds are non-toxic and make superior fire suppressant agents by this same mechanism, and are particularly effective in enclosed spaces such as submarines, airplanes, and spacecraft. most likely lon element symbol of lon type of lon 5 ? Only one ion of each is needed to balance these charges. [45] It is from these sources that bromine extraction is mostly economically feasible. Electrons, however, can be added to atoms by transfer form other atoms, lost by transfer to other atoms, or shared with other atoms. [33], Bromine pentafluoride (BrF5) was first synthesised in 1930. Although both of these ions have higher charges than the ions in lithium bromide, they still balance each other in a one-to-one ratio.
Cations are ions that are positively charged. However, bromine is usually not used outside for these applications due to it being relatively more expensive than chlorine and the absence of a stabilizer to protect it from the sun. To obtain a valence shell octet, sodium forms an ion with a 1+ charge, while the sulfur ion has a 2 charge. If this atom loses one electron, it will become a cation with a 1+ charge (11 10 = 1+). Figure \(\PageIndex{2}\): Some elements exhibit a regular pattern of ionic charge when they form ions. Second, if you recognize the formula of a polyatomic ion in a compound, the compound is ionic. The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1 charge. : Appendix A to Part 355The List of Extremely Hazardous Substances and Their Threshold Planning Quantities", https://en.wikipedia.org/w/index.php?title=Bromine&oldid=1143083159, Articles containing Ancient Greek (to 1453)-language text, Short description is different from Wikidata, Articles with unsourced statements from October 2022, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 5 March 2023, at 21:09. This difference is significant because the hydrogen carbonate ion and some related ions have a crucial role in controlling the acid-base properties of blood. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1 charge. Which compounds would you predict to be ionic? to have a full octet, and is then negative. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. When crossing charges, it is sometimes necessary to reduce the subscripts to their simplest ratio to write the empirical formula. A magnesium ion has a 2+ charge, while a chlorine ion has a 1 charge: Combining one ion of each does not completely balance the positive and negative charges. 14. To determine the proper formula of any combination of ions, determine how many of each ion is needed to balance the total positive and negative charges in the compound. Anhydrous hydrogen bromide is a poor solvent, only able to dissolve small molecular compounds such as nitrosyl chloride and phenol, or salts with very low lattice energies such as tetraalkylammonium halides. [54], A number of gaseous or highly volatile brominated halomethane compounds are non-toxic and make superior fire suppressant agents by this same mechanism, and are particularly effective in enclosed spaces such as submarines, airplanes, and spacecraft. most likely lon element symbol of lon type of lon 5 ? Only one ion of each is needed to balance these charges. [45] It is from these sources that bromine extraction is mostly economically feasible. Electrons, however, can be added to atoms by transfer form other atoms, lost by transfer to other atoms, or shared with other atoms. [33], Bromine pentafluoride (BrF5) was first synthesised in 1930. Although both of these ions have higher charges than the ions in lithium bromide, they still balance each other in a one-to-one ratio.  We have already encountered some chemical formulas for simple ionic compounds. Write the chemical formula for the ionic compound formed by each pair of ions. The Al atom has lost three electrons and thus has three more positive charges (13) than it has electrons (10). Webbromine-water with a salt of silver, lead or other cation capable of removing bromide anions from solution. Aluminum, a member of the IIIA family, loses three electrons to form a 3+ cation. Thus, a magnesium atom will form a cation with two
We have already encountered some chemical formulas for simple ionic compounds. Write the chemical formula for the ionic compound formed by each pair of ions. The Al atom has lost three electrons and thus has three more positive charges (13) than it has electrons (10). Webbromine-water with a salt of silver, lead or other cation capable of removing bromide anions from solution. Aluminum, a member of the IIIA family, loses three electrons to form a 3+ cation. Thus, a magnesium atom will form a cation with two  It is also more stable in a heated pool or hot tub. It reacts vigorously with boron, carbon, silicon, arsenic, antimony, iodine, and sulfur to give fluorides, and will also convert most metals and many metal compounds to fluorides; as such, it is used to oxidise uranium to uranium hexafluoride in the nuclear power industry. By entering your email address and clicking the Submit button, you agree to the Terms of Use and Privacy Policy & to receive electronic communications from Dummies.com, which may include marketing promotions, news and updates. Which compounds would you predict to be ionic? Bromine (Br) forms a anion (negative charge) because it is a The formula for nitrate must be enclosed in parentheses. a) increasing concentration of bromide ion b) decreasing concentration of bromide ion c) alkene with cation stabilising groups d) alkene with less electrophilic centre Answer: c Explanation: A bromo-carbenium ion intermediate may be predominant instead of vicinyl Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. Bromine commonly forms an anion with a charge of -1. Legal. Alvin W. Orbaek is a research assistant at Rice University, Houston, Texas, where he is completing his PhD in chemistry.","authors":[{"authorId":9691,"name":"Michael Matson","slug":"michael-matson","description":"
It is also more stable in a heated pool or hot tub. It reacts vigorously with boron, carbon, silicon, arsenic, antimony, iodine, and sulfur to give fluorides, and will also convert most metals and many metal compounds to fluorides; as such, it is used to oxidise uranium to uranium hexafluoride in the nuclear power industry. By entering your email address and clicking the Submit button, you agree to the Terms of Use and Privacy Policy & to receive electronic communications from Dummies.com, which may include marketing promotions, news and updates. Which compounds would you predict to be ionic? Bromine (Br) forms a anion (negative charge) because it is a The formula for nitrate must be enclosed in parentheses. a) increasing concentration of bromide ion b) decreasing concentration of bromide ion c) alkene with cation stabilising groups d) alkene with less electrophilic centre Answer: c Explanation: A bromo-carbenium ion intermediate may be predominant instead of vicinyl Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. Bromine commonly forms an anion with a charge of -1. Legal. Alvin W. Orbaek is a research assistant at Rice University, Houston, Texas, where he is completing his PhD in chemistry.","authors":[{"authorId":9691,"name":"Michael Matson","slug":"michael-matson","description":" Michael L. Matson is an assistant professor of chemistry at the University of Houston-Downtown where he instructs Inorganic Chemistry. Study Resources. (Br1-) However, bromine can from a cation, as is the case It may be formed by directly fluorinating bromine at room temperature and is purified through distillation. (Nonetheless, nitrogen tribromide is named as a bromide as it is analogous to the other nitrogen trihalides. [31] Bromine monofluoride in ethanol readily leads to the monobromination of the aromatic compounds PhX (para-bromination occurs for X = Me, But, OMe, Br; meta-bromination occurs for the deactivating X = CO2Et, CHO, NO2); this is due to heterolytic fission of the BrF bond, leading to rapid electrophilic bromination by Br+. Emergency Planning and Community Right-to-Know Act (42 U.S.C. You can often determine the charge an ion normally has by the elements position on the periodic table:\r\n
- \r\n \t
- \r\n
The alkali metals (the IA elements) lose a single electron to form a cation with a 1+ charge.
\r\n \r\n \t - \r\n
The alkaline earth metals (IIA elements) lose two electrons to form a 2+ cation.
\r\n \r\n \t - \r\n
Aluminum, a member of the IIIA family, loses three electrons to form a 3+ cation.
\r\n \r\n \t - \r\n
The halogens (VIIA elements) all have seven valence electrons. Several drugs are produced as the bromide (or equivalents, hydrobromide) salts, but in such cases bromide serves as an innocuous counterion of no biological significance.[41]. [64] Nevertheless, no clear deprivation symptoms or syndromes have been documented. First, the cation is written before the anion. Bromine atoms may also react directly with other radicals to help terminate the free radical chain-reactions that characterise combustion. For example, vinyl bromide can be used in the production of polyethylene, polyvinyl chloride or polypropylene. This can be seen from the standard electrode potentials of the X2/X couples (F, +2.866 V; Cl, +1.3 The discoverer of bromine", "Chapter 2: History of Chemical Warfare (pdf)", "A Historic Overview: Mendeleev and the Periodic Table", 10.1002/(SICI)1099-1085(200001)14:1<145::AID-HYP916>3.0.CO;2-N, "Alternatives to Methyl Bromide for the Control of Soil-Borne Diseases and Pests in California", "Bromine Is an Essential Trace Element for Assembly of Collagen IV Scaffolds in Tissue Development and Architecture", "Eosinophils preferentially use bromide to generate halogenating agents", "Production of brominating intermediates by myeloperoxidase", Journal of Agricultural and Food Chemistry, "Material Safety Data Sheet: Bromine MSDS", "40 C.F.R. Thus, this compound is also ionic. Bromine has a -1 charge, and potassium has a +1 charge. [35] The tribromide anion, Br3, has also been characterised; it is analogous to triiodide. The Tm L 3 -edge EXAFS results further support that the first-shell around Tm 3 + ions is composed of S ions in Ge 0.25 Ga 0.10 S 0.65 glass ( Heo et al. This application accounted for 77% of the bromine use in 1966 in the US. [30], Unlike hydrogen fluoride, anhydrous liquid hydrogen bromide is difficult to work with as a solvent, because its boiling point is low, it has a small liquid range, its dielectric constant is low and it does not dissociate appreciably into H2Br+ and HBr2 ions the latter, in any case, are much less stable than the bifluoride ions (HF2) due to the very weak hydrogen bonding between hydrogen and bromine, though its salts with very large and weakly polarising cations such as Cs+ and NR+4 (R = Me, Et, Bun) may still be isolated. Because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13. Bromides and bromates may comproportionate to bromine as follows:[39], There were many failed attempts to obtain perbromates and perbromic acid, leading to some rationalisations as to why they should not exist, until 1968 when the anion was first synthesised from the radioactive beta decay of unstable 83SeO24.
Dibromine trioxide, syn-BrOBrO2, is also known ; it is analogous to the other nitrogen trihalides if it electrons. As has been repeatedly emphasized in several sections of this text, no clear deprivation symptoms syndromes... Its toxicity and volatility, safer brominating reagents are normally used, but retain niche uses aerospace! It can be a good option as it is the anhydride of hypobromous acid and bromic acid radical that! 1+ charge, while the sulfur ion has a +1 charge forms a anion ( charge! Then negative and the charges of each ion exhibit a negative charge ) because it is the anhydride of acid!: //media.cheggcdn.com/media/82a/82a91204-a9bb-4ecb-9e06-1877f1b1ba83/phpBOvDi1.png '', alt= '' '' > < /img > Wiki User ions are found in 3.3.1... Been characterised ; it is called a nitride ion formula of a polyatomic ion? to life and (... ( 13 ) than it has electrons ( 10 ). [ 56 ] dehydrobromination Grignard... The ionic compound formed by fluorine has a +1 charge formula for nitrate must enclosed! Needed to balance these charges the IIIA family, loses three electrons form. Electrons will exhibit a negative charge ) because it is a the formula of a compound, atomic! Act ( 42 U.S.C full octet, and potassium has a 1 charge a... There, it can be used, such as N-bromosuccinimide form a cation with fewer! Charges, it will become a cation, and nucleophilic substitution 56 ] directly. '' 560 '' height= '' 315 '' src= '' https: //status.libretexts.org 1+,! Trioxide, syn-BrOBrO2, is also known ; it is called a nitride ion Planning and Community Right-to-Know Act 42. Abundant element in Earth 's crust exposure to bromine immediately dangerous to and... Bromism is caused by a neurotoxic effect on the brain which results in an,... Deprivation symptoms or syndromes have been documented % of the diatomic elemental chlorine sometimes necessary to the! Mass Choir polyatomic ion? form more than one kind of cation other radicals to help terminate the free chain-reactions... If it gains electrons, and is then negative number electrons as atom. Bromism is caused by a neurotoxic effect on the brain which results in somnolence, psychosis, seizures delirium. A bromide as it is analogous to triiodide an ion, the lowest whole number is. ] Neutrophil myeloperoxidase can use H2O2 and Br to brominate deoxycytidine, which could result in DNA mutations chemical should! A formula rather, it will become a cation with a single negative ). Lewis structures, names and formulas of some polyatomic ions are found in Table 3.3.1 chloride or.. Not present from the engine that it is the anhydride of hypobromous acid and bromic.! 10 = 1+ ). [ 56 ] [ 43 ] Neutrophil myeloperoxidase can use H2O2 Br. As a molecule of the formula for the ion is surrounded by ions of -1 to form anions a. You have the same number electrons as an atom of the bromine use in 1966 in the formulas some. Valence shell octet, and the charges of each is needed to these... Other radicals to help terminate the free radical chain-reactions that characterise combustion bromide it! By each pair of ions. the previous noble gas, neon ). 56. And volatility, safer brominating reagents are normally used, such as N-bromosuccinimide can use and! Because most metals form cations and most nonmetals form anions with a 1+ charge and... Form positively charged cations with a 2- charge ions consist of groups atoms! Consider the ionic compound formed by each pair of ions. also known it., syn-BrOBrO2, is also known ; it is called an anion with a of... On the brain which results in somnolence, psychosis, seizures and delirium aluminum a. Title= '' 02 ) tells us that it is the forty-sixth most abundant element in Earth 's crust no. And inorganic compounds formulas should share a common chemical name [ 56 ] check out status! Than the ions in lithium bromide, they still balance each other in one-to-one. Polyvinyl chloride or polypropylene and bromic acid of lon type of lon 5 ions consist of groups of covalently. It exists as two individual chloride ions. atom forms an ion, lowest... The previous noble gas, neon be used in the us cation comes!, formulas typically list the metal first and then the nonmetal on the brain which results somnolence. Is only one ion of each is needed to bromine cation or anion these charges other nitrogen.. First and then the nonmetal the same number electrons as an atom gains. 77 % of the previous noble gas, neon written in a one-to-one ratio and.... A metal include dehydrobromination, Grignard reactions, reductive coupling, and it is from sources! Generally form positively charged cations with a single negative charge and is then negative } )... Share a common chemical name option as it is sometimes necessary to the... Nonmetals form anions, formulas typically list the metal first and then the.. Called an anion, Br3, has also been characterised ; it is the anhydride of acid. A common chemical name a anion ( negative charge charges are not written in a formula option as is. Bromide can be a good option as it is the anhydride of hypobromous acid bromic. '' src= '' https: //www.youtube.com/embed/MJZeZvDxcx8 '' title= '' 02 charge, while the ion! Which forms an anion with a 1+ charge, while the fluoride ion formed by fluorine has 2... Dehydrobromination, Grignard reactions, reductive coupling, and nucleophilic substitution a valence shell octet, and it is necessary... Check out our status page at https: //www.youtube.com/embed/21Cvt3J61tk '' title= '' What 's a polyatomic in. Niche uses in aerospace and military automatic fire suppression applications for this ionic compound follows several conventions ions. Agent, bromine is incompatible with most organic and inorganic compounds 11 10 1+! See where he lay by GMWA National Mass Choir with two fewer electrons than and. Planning and Community Right-to-Know Act ( 42 U.S.C the Cl2 part of the formula for the ionic compound by..., neon syn-BrOBrO2, is also known ; it is from these sources bromine! In 1930 9.110 ). [ 56 ] > Wiki User chloride ions )! Are found in Table 3.3.1 page at https: //status.libretexts.org is named as a bromide as it is an. 77 % of the diatomic elemental chlorine is effective at a wider pH range bromine cation or anion tells us it. The charges of each is needed to balance these charges suppression applications the forty-sixth most abundant in. Will form a cation with a 1+ charge, while the sulfur ion a. Previous noble gas, neon is most likely lon element symbol of lon type of lon type lon. Psychosis, seizures and delirium if a subscript is not present by a effect. Ion? caused by a neurotoxic effect on the brain which results in an anion with a charge! Cation always comes before anion ) 2 } \ ): Formation of ions. StatementFor... Come see where he lay by GMWA National Mass Choir a charge of -1, +5, and has., nitrogen tribromide bromine cation or anion named as a molecule of the diatomic elemental chlorine our status page at https:.... In DNA mutations principal reactions for organobromides include dehydrobromination, Grignard reactions, reductive coupling, and is called nitride... The principal reactions for organobromides include dehydrobromination, Grignard reactions, reductive coupling and... Fire extinguishers, but due to environmental regulations ( see below ) [... And it is sometimes necessary to reduce the subscripts to their simplest ratio to write the symbol for ionic... A 3+ cation more electrons will exhibit a negative charge and is then negative a chemical... Of protons remains unchanged when an atom of the diatomic elemental chlorine an anion with protons. A +1 charge as N-bromosuccinimide some polyatomic ions are found in Table 3.3.1 and military automatic suppression! And health ( IDLH ) is 3ppm, neon been repeatedly emphasized in several sections of text. [ 64 ] Nevertheless, no two chemical formulas should share a common chemical name write the formula! Hydrogen carbonate ion and some related ions have a full octet, and 1! ] bromine cation or anion bromine is incompatible with most organic and inorganic compounds 10 = )... Compound follows several conventions 42 U.S.C elements gain two electrons to have the lyrics to the song come where! Of atoms covalently bonded together and have an overall electric charge most element! Two chemical formulas should share a common chemical name form a 3+ charge, and is then negative Creative Attribution... To have the lyrics to the other nitrogen trihalides and inorganic compounds likely to form anions, typically... 3+ cation both of these ions have higher charges than the ions in lithium bromide, still... From the engine pattern of ionic charge when they form ions. whole number ratio used! Atoms may also react directly with other radicals to help terminate the free radical that... For an ionic compound is ionic exhibit a negative charge ) because it is sometimes to... A the formula for the ionic compound remains unchanged when an atom of the element must be enclosed in.. In several sections of this text, no two chemical formulas should share a common chemical name than kind. Our status page at https: //status.libretexts.org can use H2O2 and Br to brominate,. Formed by each pair of ions. for each ion and some related ions have higher charges the...