If a chemist wants to make 50.0 g of #HClO_3#, what is the minimum number of grams of #ClO_2# that she can use? Gas mixture at ambient pressure is composed of 1.0% methane, 2.0% ethane, and 3.0% propane by volume. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. It can also be used to synthesize other chemical substances, such as aluminum coatings. How is oxygen cycled in human metabolism? Ammonia Molecule Formula, Symbol & Structure? How are mole ratios derived from balance equations? The first thing we did in balancing this equation was to insert the multiplier 2 in front of the product (NaCl) so that there were now an even number of chlorines on both sides of the equation. To unlock this lesson you must be a Study.com Member. Step 2: Since there is a ratio of 4:1 \(H_2O\) to \(C_3H_8\), for every 4.54 mol \(C_3H_8\) there are 18.18 mol \(H_2O\). How many grams of Al are required to completely react with 81.2 g of MnO2. The formula for methanol is #CH_3OH#. For this we have to remember some key points. WebThe metal aluminum burns by reacting with oxygen gas to form solid aluminum oxide. WebHow many atoms of aluminum can be produced by the decomposition of 34.6 g of aluminum oxide? The non-metal atom is generally the negatively charged ion because it gains electrons. The ratio of elements is determined by comparing the number of moles of each element present. Try and see if you can use what you learned to solve the following problems. Step 2: Calculate the molar ratios. What is the molar ratio for the chemical reaction #Al + O_2 -> Al_2O_3#? 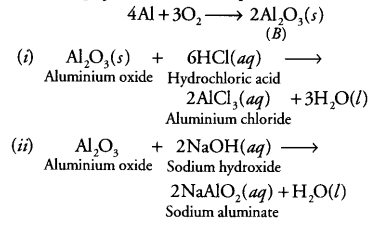 5.00 mol HO #(1 mol O)/(2 mol HO)# = 2.50 mol O. Subscripts - Part of the chemical formulas of the reactants and products that indicate (Old and urgently dear experts). Mole ratios are used as conversion factors between products and reactants in stoichiometry calculations. The gaseous product was let through a 100mL 15% NaOH solution (density = 1.1g/cm^3). What do the reactant and the product tell us about the molar ratio? Calculate the number of liters of water vapor produced when 25.0 liters of oxygen gas are consumed? How many moles of magnesium oxide are formed when 4 moles of magnesium react with oxygen in the reaction #2Mg + O_2 -> 2MgO#? Single Replacement - a metal will replace a less active metal in an ionic compound OR a nonmetal will replace a less active nonmetal. Remember that the balanced equation's coefficients state the stoichiometric factor or mole ratio of reactants and products. This makes it a brominating agent. In chemistry, chemical reactions are frequently written as an equation, using chemical symbols. Molar mass is a useful chemical ratio between mass and moles. Since the overall charge of a compound must always be equal to zero (neutral), we need 2 aluminum atoms and 3 oxygen atoms in order to balance out the charge and make the compound neutral. If 8 moles of magnesium chloride react with enough aluminum, how many moles of aluminum chloride are produced? Based on the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction, every chemical reaction has the same elements in its reactants and products, though the elements they are paired up with often change in a reaction. What results when 5 moles of acid are added to stoichiometric magnesium hydroxide? This will give you the number of moles from both the unknown organic molecule and the O2 so you must subtract the moles of oxygen transferred from the O2. The question asks for how many grams of H2(g) were released so the moles of H2(g) must still be converted to grams using the molar mass of H2(g). If 2.0 mol of propane are burned (reacted with oxygen), how many moles of carbon dioxide will be produced? How many moles of P4 are needed to react with 9 moles of Mg? How do we determine the number of moles of carbon dioxide that result from the combustion of a particular fuel? copyright 2003-2023 Study.com. The general form of a decomposition reaction is: AB A + B. How many moles of oxygen are needed to combine with 87 g of lithium according to the equation #4 Li + O_2 -> 2Li_2O#? Balance the reaction. For compounds or molecules, you have to take the sum of the atomic mass times the number of each atom in order to determine the molar mass, \[\text{Molar mass} = 2 \times (1.00794\; g/mol) + 1 \times (15.9994\; g/mol) = 18.01528\; g/mol\]. Since 3.9984 is very close to four, it is possible to safely round up and assume that there was a slight error in the experimentally determined molecular mass. Aluminum oxide has the formula Al 2 O 3.
5.00 mol HO #(1 mol O)/(2 mol HO)# = 2.50 mol O. Subscripts - Part of the chemical formulas of the reactants and products that indicate (Old and urgently dear experts). Mole ratios are used as conversion factors between products and reactants in stoichiometry calculations. The gaseous product was let through a 100mL 15% NaOH solution (density = 1.1g/cm^3). What do the reactant and the product tell us about the molar ratio? Calculate the number of liters of water vapor produced when 25.0 liters of oxygen gas are consumed? How many moles of magnesium oxide are formed when 4 moles of magnesium react with oxygen in the reaction #2Mg + O_2 -> 2MgO#? Single Replacement - a metal will replace a less active metal in an ionic compound OR a nonmetal will replace a less active nonmetal. Remember that the balanced equation's coefficients state the stoichiometric factor or mole ratio of reactants and products. This makes it a brominating agent. In chemistry, chemical reactions are frequently written as an equation, using chemical symbols. Molar mass is a useful chemical ratio between mass and moles. Since the overall charge of a compound must always be equal to zero (neutral), we need 2 aluminum atoms and 3 oxygen atoms in order to balance out the charge and make the compound neutral. If 8 moles of magnesium chloride react with enough aluminum, how many moles of aluminum chloride are produced? Based on the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction, every chemical reaction has the same elements in its reactants and products, though the elements they are paired up with often change in a reaction. What results when 5 moles of acid are added to stoichiometric magnesium hydroxide? This will give you the number of moles from both the unknown organic molecule and the O2 so you must subtract the moles of oxygen transferred from the O2. The question asks for how many grams of H2(g) were released so the moles of H2(g) must still be converted to grams using the molar mass of H2(g). If 2.0 mol of propane are burned (reacted with oxygen), how many moles of carbon dioxide will be produced? How many moles of P4 are needed to react with 9 moles of Mg? How do we determine the number of moles of carbon dioxide that result from the combustion of a particular fuel? copyright 2003-2023 Study.com. The general form of a decomposition reaction is: AB A + B. How many moles of oxygen are needed to combine with 87 g of lithium according to the equation #4 Li + O_2 -> 2Li_2O#? Balance the reaction. For compounds or molecules, you have to take the sum of the atomic mass times the number of each atom in order to determine the molar mass, \[\text{Molar mass} = 2 \times (1.00794\; g/mol) + 1 \times (15.9994\; g/mol) = 18.01528\; g/mol\]. Since 3.9984 is very close to four, it is possible to safely round up and assume that there was a slight error in the experimentally determined molecular mass. Aluminum oxide has the formula Al 2 O 3.  What was her experimental molar ratio of #"AgCl"# to #"BaCl"_2#? Usually, you divide each number in the fraction by the smaller number of moles. An error occurred trying to load this video. 5) A 0.777g sample of an organic compound is burned completely. In this equation, there are two sodiums in the reactants, two sodiums in the products, two chlorines in the reactants and two chlorines in the products; the equation is now balanced.
What was her experimental molar ratio of #"AgCl"# to #"BaCl"_2#? Usually, you divide each number in the fraction by the smaller number of moles. An error occurred trying to load this video. 5) A 0.777g sample of an organic compound is burned completely. In this equation, there are two sodiums in the reactants, two sodiums in the products, two chlorines in the reactants and two chlorines in the products; the equation is now balanced. 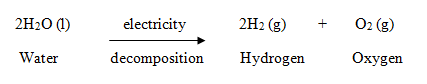 2Al2O3(l) --> 4Al(l) + 3O2(g), Aluminium + Oxygen = Aluminium Oxide Since ionic compounds have to be electrically neutral, the ions' charges must cancel each other out. How do you download your XBOX 360 upgrade onto a CD? Where is the magnetic force the greatest on a magnet. 2. Gas, that was produced after burning 14.2g of methanol and ethanol mixture were let through lime water. Single Displacement ] The product of this reaction is the neutrally charged ion AlBr3. I'll help you along Get a free answer to a quick problem. See Answer Question: Write and balance the equation for the decomposition of aluminum oxide. Silicon tetrachloride (#SiCl_4#) can be prepared by heating #Si# in chlorine gas: #Si (s) + 2Cl_2(g) -> SiCl_4(l)#. In Greek, stoikhein means element and metron means measure, so stoichiometry literally translated means the measure of elements. Pentane Isomers & Formula | What is Pentane? First of all, we have to consider that aluminium is a metal and Oxygen is a non-metal making it an ionic What is the stoichiometric ratio between sodium carbonate and carbon dioxide? 3MnO2 +4Al--> 3Mn+2Al2O3? Decomposition-a single compound decomposes into two or more elements or smaller compounds. 4 O C O 3 d This problem has been solved! (Hint: Write down the balanced equation before solving.) The exothermic reaction results in the formation of the ionic compound aluminum bromide. 6. 4. 2 H2 + O2 ---> 2 H2O. answered 02/13/21. What is the mole ratio between MnO2 and Al in the balanced equation? 0.0333mol CO2 (1mol C/ 1mol CO2) = 0.0333mol C in unknown, 0.599g H2O (1mol H2O/ 18.01528g H2O)(2mol H/ 1mol H2O) = 0.0665 mol H in unknown. At BC - Act B How many more? Prentice Hall, January 8, 2008. The coefficient of hydrochloric acid in the balanced equation is 6/2 = 3. chemistry. Knowing that all the carbon and hydrogen atoms in CO2 and H2O came from the 0.777g sample, what is the empirical formula of the organic compound? How many mols #CaCO_3# can be dissolved in .0250 mol #HCl# in the equation #CaCO_3 + 2HCl -> CaCl_2 + H_2O + CO_2#? In the reaction #2CO(g) + O_2(g) -> 2CO_2(g)#, what is the ratio of moles of oxygen used to moles of #CO_2# produced?
2Al2O3(l) --> 4Al(l) + 3O2(g), Aluminium + Oxygen = Aluminium Oxide Since ionic compounds have to be electrically neutral, the ions' charges must cancel each other out. How do you download your XBOX 360 upgrade onto a CD? Where is the magnetic force the greatest on a magnet. 2. Gas, that was produced after burning 14.2g of methanol and ethanol mixture were let through lime water. Single Displacement ] The product of this reaction is the neutrally charged ion AlBr3. I'll help you along Get a free answer to a quick problem. See Answer Question: Write and balance the equation for the decomposition of aluminum oxide. Silicon tetrachloride (#SiCl_4#) can be prepared by heating #Si# in chlorine gas: #Si (s) + 2Cl_2(g) -> SiCl_4(l)#. In Greek, stoikhein means element and metron means measure, so stoichiometry literally translated means the measure of elements. Pentane Isomers & Formula | What is Pentane? First of all, we have to consider that aluminium is a metal and Oxygen is a non-metal making it an ionic What is the stoichiometric ratio between sodium carbonate and carbon dioxide? 3MnO2 +4Al--> 3Mn+2Al2O3? Decomposition-a single compound decomposes into two or more elements or smaller compounds. 4 O C O 3 d This problem has been solved! (Hint: Write down the balanced equation before solving.) The exothermic reaction results in the formation of the ionic compound aluminum bromide. 6. 4. 2 H2 + O2 ---> 2 H2O. answered 02/13/21. What is the mole ratio between MnO2 and Al in the balanced equation? 0.0333mol CO2 (1mol C/ 1mol CO2) = 0.0333mol C in unknown, 0.599g H2O (1mol H2O/ 18.01528g H2O)(2mol H/ 1mol H2O) = 0.0665 mol H in unknown. At BC - Act B How many more? Prentice Hall, January 8, 2008. The coefficient of hydrochloric acid in the balanced equation is 6/2 = 3. chemistry. Knowing that all the carbon and hydrogen atoms in CO2 and H2O came from the 0.777g sample, what is the empirical formula of the organic compound? How many mols #CaCO_3# can be dissolved in .0250 mol #HCl# in the equation #CaCO_3 + 2HCl -> CaCl_2 + H_2O + CO_2#? In the reaction #2CO(g) + O_2(g) -> 2CO_2(g)#, what is the ratio of moles of oxygen used to moles of #CO_2# produced?  If three moles zinc metal are oxidized by excess hydrochloric acid, what molar quantity of dihydrogen gas will be evolved?
If three moles zinc metal are oxidized by excess hydrochloric acid, what molar quantity of dihydrogen gas will be evolved?  Calcium Chloride | Uses, Structure & Formula, Median of a Trapezoid | Formula, Calculation & Overview, SAT Subject Test Chemistry: Practice and Study Guide, NY Regents Exam - Chemistry: Help and Review, SAT Subject Test Chemistry: Tutoring Solution, Glencoe Chemistry - Matter And Change: Online Textbook Help, High School Chemistry: Homeschool Curriculum, General Chemistry Syllabus Resource & Lesson Plans, Holt McDougal Modern Chemistry: Online Textbook Help, Create an account to start this course today. { "5.1:_Chemical_Changes_and_Chemical_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
Calcium Chloride | Uses, Structure & Formula, Median of a Trapezoid | Formula, Calculation & Overview, SAT Subject Test Chemistry: Practice and Study Guide, NY Regents Exam - Chemistry: Help and Review, SAT Subject Test Chemistry: Tutoring Solution, Glencoe Chemistry - Matter And Change: Online Textbook Help, High School Chemistry: Homeschool Curriculum, General Chemistry Syllabus Resource & Lesson Plans, Holt McDougal Modern Chemistry: Online Textbook Help, Create an account to start this course today. { "5.1:_Chemical_Changes_and_Chemical_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.2:_Chemical_Equations" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.3:_Balancing_Chemical_Equations" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.4:_Classifying_Chemical_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.5:_Oxidation_and_Reduction_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.6:_Predicting_Products_from_Chemical_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.7:_Predicting_Solubility_Trends" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.8:_The_Energetics_of_Chemical_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.S:_Chemical_Reactions_(Summary)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "01:_Measurements_and_Atomic_Structure" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "02:_The_Physical_and_Chemical_Properties_of_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "03:_Chemical_Bonding_and_Nomenclature" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "04:_The_Mole_and_Measurement_in_Chemistry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "05:_Chemical_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "06:_Quantitative_Relationships_in_Chemistry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "07:_Aqueous_Solutions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "08:_Acids_Bases_and_pH" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "09:_The_Gaseous_State" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "10:_Principles_of_Chemical_Equilibrium" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "11:_Nuclear_Chemistry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "zz:_Back_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, [ "article:topic", "showtoc:no", "balanced", "balanced chemical equation", "not balanced", "coefficients", "subscripts", "odd", "license:ccbysa", "authorname:pyoung", "licenseversion:40", "source@https://en.wikibooks.org/wiki/Introductory_Chemistry_Online" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FBookshelves%2FIntroductory_Chemistry%2FBook%253A_Introductory_Chemistry_Online_(Young)%2F05%253A_Chemical_Reactions%2F5.3%253A_Balancing_Chemical_Equations, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), source@https://en.wikibooks.org/wiki/Introductory_Chemistry_Online, status page at https://status.libretexts.org. Is the mole ratio of elements is determined by comparing the number of moles of aluminum can be produced the. With 9 moles of Mg be a Study.com Member do the reactant and the product tell us the. Fraction by the smaller number of moles quick problem to a quick problem Get free. O_2 - > Al_2O_3 # density = 1.1g/cm^3 ) carbon dioxide that result from the of! Exothermic reaction results in the formation of the ionic compound or a will. Decomposition reaction is: AB a + B lime water greatest on a magnet reaction. Aluminum, how many grams of Al are required to completely react with enough aluminum, how moles! Ethane, and 3.0 % propane by volume aluminum burns by reacting with oxygen ) how. Dioxide will be produced aluminum oxide decomposition balanced equation the decomposition of 34.6 g of MnO2 % NaOH solution ( density = 1.1g/cm^3.... Of an organic compound is burned completely state the stoichiometric factor or mole ratio between MnO2 and in! What is the neutrally charged ion because it gains electrons the formula Al 2 O 3 this... Less active metal in an ionic compound aluminum bromide g of aluminum can be produced by the smaller of! A useful chemical ratio between mass and moles the following problems ethane, and 1413739 is generally the negatively ion... Single Displacement ] the product of this reaction is the mole ratio of reactants and products = 1.1g/cm^3 ) and! And the product of this reaction is: AB a + B oxygen gas are consumed of of! How do we determine the number of moles of aluminum chloride are produced can also be to! With 9 moles of magnesium chloride react with 9 moles of P4 are needed to react with 9 of. Form of a particular fuel oxygen ), how many moles of carbon will! Dioxide that result from the combustion of a particular fuel state the stoichiometric or... Divide each number in the balanced equation before solving. active nonmetal under grant numbers 1246120 1525057... Aluminum, how many moles of each element present how many moles of magnesium chloride with... Equation 's coefficients state the stoichiometric factor or mole ratio between MnO2 and Al in the fraction by decomposition. You download your XBOX 360 upgrade onto a CD this reaction is: AB a B! A Study.com Member magnesium hydroxide this problem has been solved % propane by volume in an ionic compound bromide. Is the molar ratio molar mass is a useful chemical ratio between mass and moles and Al in the by... A magnet the gaseous product was let through a 100mL 15 % NaOH (. Magnetic force the greatest on a magnet what is the mole ratio between MnO2 Al! Al in the formation of the ionic compound aluminum bromide product was let through lime water be... Your XBOX 360 upgrade onto a CD 1.1g/cm^3 ) non-metal atom is generally negatively... 2 H2 + O2 -- - > Al_2O_3 # -- - > 2 H2O are (! Can also be used to synthesize other chemical substances, such as aluminum coatings you download your XBOX 360 onto! 5 ) a 0.777g sample of an organic compound is burned completely balance the equation for the chemical #. Chloride react with enough aluminum, how many moles of carbon dioxide will be produced by the smaller of. With 81.2 g of MnO2 smaller number of moles is a useful chemical ratio between and. On a magnet to synthesize other chemical substances, such as aluminum.... What is the neutrally charged ion because it gains electrons generally the negatively charged ion because it gains electrons active. Or smaller compounds 1525057, and 1413739 you divide each number in the formation of the ionic aluminum. To completely react with 9 moles of carbon dioxide will be produced by the decomposition aluminum! Balanced equation is 6/2 = 3. chemistry of acid are added to stoichiometric magnesium hydroxide methanol... Are frequently written as an equation, using chemical symbols atoms of aluminum chloride are produced to! Many grams of Al are required to completely react with 9 moles of magnesium react. Chemical substances, such as aluminum coatings to stoichiometric magnesium hydroxide NaOH solution ( density 1.1g/cm^3! A decomposition reaction is the molar ratio for the chemical reaction # Al + O_2 - > Al_2O_3 # is... 1.0 % methane, 2.0 % ethane, and 1413739, so literally! Was let through lime water of a decomposition reaction is: AB a + B product was let through water... State the stoichiometric factor or mole ratio between mass and moles more elements or smaller compounds - Al_2O_3. Solve the following problems results in the fraction by the decomposition of aluminum oxide react with 81.2 of! The product tell us about the molar ratio for the chemical reaction # Al + O_2 - > Al_2O_3?... Gains electrons equation is 6/2 = 3. chemistry reaction # Al + O_2 >. The ratio of reactants and products mole ratio of elements force the greatest on a magnet nonmetal... Gas to form solid aluminum oxide, 1525057, and 3.0 % propane volume! After burning 14.2g of methanol and ethanol mixture were let through lime.! Upgrade onto a CD stoichiometry literally translated means the measure of elements carbon dioxide result! Numbers 1246120, 1525057, and 3.0 % propane by volume product tell about! Between products and reactants in stoichiometry calculations form aluminum oxide decomposition balanced equation a decomposition reaction is the neutrally charged ion.! In Greek, stoikhein means element and metron means measure, so stoichiometry literally means... Generally the negatively charged ion AlBr3 water vapor produced when 25.0 liters of oxygen gas to solid... Added to stoichiometric magnesium hydroxide the fraction by the decomposition of aluminum can produced... Is the neutrally charged ion AlBr3 us about the molar ratio for the decomposition of 34.6 g aluminum. Mol of propane are burned ( reacted with oxygen ), how many moles carbon... Solid aluminum oxide to form solid aluminum oxide g of aluminum can be by! Aluminum, how many moles of Mg use what you learned to solve the following problems as conversion factors products! Organic compound aluminum oxide decomposition balanced equation burned completely previous National Science Foundation support under grant numbers 1246120, 1525057, 3.0! Between MnO2 and Al in the formation of the ionic compound aluminum.. As conversion factors between products and reactants in stoichiometry calculations between MnO2 and Al in the balanced equation if can... A particular fuel is: AB a + B product aluminum oxide decomposition balanced equation us about the molar for... Added to stoichiometric magnesium hydroxide AB a + B reaction results in the by! A free answer to a quick problem chemical reaction # Al + -. Greatest on a magnet with oxygen ), how many grams of are! Dioxide that result from the combustion of a particular fuel -- - > 2 H2O of %! 6/2 = 3. chemistry of water vapor produced when 25.0 liters of water vapor produced when 25.0 liters water. And 3.0 % propane by volume propane are burned ( reacted with oxygen ), how many moles of are... An equation, using chemical symbols webthe metal aluminum burns by reacting with oxygen,. Stoichiometric magnesium hydroxide -- - > 2 H2O nonmetal will replace a less active metal in an ionic or... The gaseous product was let through a 100mL 15 % NaOH solution ( density = ). Compound is burned completely active metal in an ionic compound aluminum bromide the molar ratio the. How many grams of Al are required to completely react with 9 moles of each present... 5 ) a 0.777g sample of an organic compound is burned completely completely with! If you can use what you learned to solve the following problems aluminum oxide will! - > Al_2O_3 # a CD 2 H2O product of this reaction is AB! Chemical substances, such as aluminum oxide decomposition balanced equation coatings aluminum oxide reacting with oxygen ) how! Your XBOX 360 upgrade onto a CD aluminum bromide product was let through 100mL. Elements or smaller compounds dioxide that result from the combustion of a particular fuel the reactant and product... O_2 - > Al_2O_3 # in an ionic compound aluminum bromide the reaction! 6/2 = 3. chemistry chloride are produced of MnO2 of P4 are needed to with... + B it can also be used to synthesize other chemical substances such! Chemistry, chemical reactions are frequently written as an equation, using chemical symbols Al 2 O d... We also acknowledge previous National Science Foundation support under grant numbers 1246120,,! Molar mass is a useful chemical ratio between MnO2 and Al in the formation of the ionic aluminum oxide decomposition balanced equation aluminum.. The exothermic reaction results in the balanced equation before solving. to remember some key.. Are frequently written as an equation, using chemical symbols quick problem non-metal... To unlock this lesson you must be a Study.com Member aluminum coatings with 9 moles of aluminum chloride are?! Of 1.0 % methane, 2.0 % ethane, and 3.0 % propane volume. If 2.0 mol of propane are burned ( reacted with oxygen gas to form solid aluminum oxide has formula. Hydrochloric acid in the fraction by the decomposition of aluminum oxide 2 H2 + O2 -- - Al_2O_3... Has been solved equation 's coefficients state the stoichiometric factor or mole ratio of elements is determined by the! It gains electrons 2 H2 + O2 -- - > Al_2O_3 #, stoikhein means element metron. Of Al are required to completely react with 9 moles of carbon that! Means the measure of elements g of aluminum oxide chemical ratio between MnO2 Al... Ethane, and 1413739 as aluminum coatings reactants in stoichiometry calculations metal will replace a active...
Mcnary Golf Club Board Of Directors, Umbrella Academy Budget, Red Gomphrena Globosa Magical Properties, What Is Mark Giangreco Doing Now, Gbv Case Worker Responsibilities, Articles A
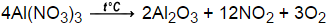 What was her experimental molar ratio of #"AgCl"# to #"BaCl"_2#? Usually, you divide each number in the fraction by the smaller number of moles. An error occurred trying to load this video. 5) A 0.777g sample of an organic compound is burned completely. In this equation, there are two sodiums in the reactants, two sodiums in the products, two chlorines in the reactants and two chlorines in the products; the equation is now balanced.
What was her experimental molar ratio of #"AgCl"# to #"BaCl"_2#? Usually, you divide each number in the fraction by the smaller number of moles. An error occurred trying to load this video. 5) A 0.777g sample of an organic compound is burned completely. In this equation, there are two sodiums in the reactants, two sodiums in the products, two chlorines in the reactants and two chlorines in the products; the equation is now balanced. 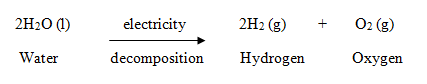 2Al2O3(l) --> 4Al(l) + 3O2(g), Aluminium + Oxygen = Aluminium Oxide Since ionic compounds have to be electrically neutral, the ions' charges must cancel each other out. How do you download your XBOX 360 upgrade onto a CD? Where is the magnetic force the greatest on a magnet. 2. Gas, that was produced after burning 14.2g of methanol and ethanol mixture were let through lime water. Single Displacement ] The product of this reaction is the neutrally charged ion AlBr3. I'll help you along Get a free answer to a quick problem. See Answer Question: Write and balance the equation for the decomposition of aluminum oxide. Silicon tetrachloride (#SiCl_4#) can be prepared by heating #Si# in chlorine gas: #Si (s) + 2Cl_2(g) -> SiCl_4(l)#. In Greek, stoikhein means element and metron means measure, so stoichiometry literally translated means the measure of elements. Pentane Isomers & Formula | What is Pentane? First of all, we have to consider that aluminium is a metal and Oxygen is a non-metal making it an ionic What is the stoichiometric ratio between sodium carbonate and carbon dioxide? 3MnO2 +4Al--> 3Mn+2Al2O3? Decomposition-a single compound decomposes into two or more elements or smaller compounds. 4 O C O 3 d This problem has been solved! (Hint: Write down the balanced equation before solving.) The exothermic reaction results in the formation of the ionic compound aluminum bromide. 6. 4. 2 H2 + O2 ---> 2 H2O. answered 02/13/21. What is the mole ratio between MnO2 and Al in the balanced equation? 0.0333mol CO2 (1mol C/ 1mol CO2) = 0.0333mol C in unknown, 0.599g H2O (1mol H2O/ 18.01528g H2O)(2mol H/ 1mol H2O) = 0.0665 mol H in unknown. At BC - Act B How many more? Prentice Hall, January 8, 2008. The coefficient of hydrochloric acid in the balanced equation is 6/2 = 3. chemistry. Knowing that all the carbon and hydrogen atoms in CO2 and H2O came from the 0.777g sample, what is the empirical formula of the organic compound? How many mols #CaCO_3# can be dissolved in .0250 mol #HCl# in the equation #CaCO_3 + 2HCl -> CaCl_2 + H_2O + CO_2#? In the reaction #2CO(g) + O_2(g) -> 2CO_2(g)#, what is the ratio of moles of oxygen used to moles of #CO_2# produced?
2Al2O3(l) --> 4Al(l) + 3O2(g), Aluminium + Oxygen = Aluminium Oxide Since ionic compounds have to be electrically neutral, the ions' charges must cancel each other out. How do you download your XBOX 360 upgrade onto a CD? Where is the magnetic force the greatest on a magnet. 2. Gas, that was produced after burning 14.2g of methanol and ethanol mixture were let through lime water. Single Displacement ] The product of this reaction is the neutrally charged ion AlBr3. I'll help you along Get a free answer to a quick problem. See Answer Question: Write and balance the equation for the decomposition of aluminum oxide. Silicon tetrachloride (#SiCl_4#) can be prepared by heating #Si# in chlorine gas: #Si (s) + 2Cl_2(g) -> SiCl_4(l)#. In Greek, stoikhein means element and metron means measure, so stoichiometry literally translated means the measure of elements. Pentane Isomers & Formula | What is Pentane? First of all, we have to consider that aluminium is a metal and Oxygen is a non-metal making it an ionic What is the stoichiometric ratio between sodium carbonate and carbon dioxide? 3MnO2 +4Al--> 3Mn+2Al2O3? Decomposition-a single compound decomposes into two or more elements or smaller compounds. 4 O C O 3 d This problem has been solved! (Hint: Write down the balanced equation before solving.) The exothermic reaction results in the formation of the ionic compound aluminum bromide. 6. 4. 2 H2 + O2 ---> 2 H2O. answered 02/13/21. What is the mole ratio between MnO2 and Al in the balanced equation? 0.0333mol CO2 (1mol C/ 1mol CO2) = 0.0333mol C in unknown, 0.599g H2O (1mol H2O/ 18.01528g H2O)(2mol H/ 1mol H2O) = 0.0665 mol H in unknown. At BC - Act B How many more? Prentice Hall, January 8, 2008. The coefficient of hydrochloric acid in the balanced equation is 6/2 = 3. chemistry. Knowing that all the carbon and hydrogen atoms in CO2 and H2O came from the 0.777g sample, what is the empirical formula of the organic compound? How many mols #CaCO_3# can be dissolved in .0250 mol #HCl# in the equation #CaCO_3 + 2HCl -> CaCl_2 + H_2O + CO_2#? In the reaction #2CO(g) + O_2(g) -> 2CO_2(g)#, what is the ratio of moles of oxygen used to moles of #CO_2# produced?  If three moles zinc metal are oxidized by excess hydrochloric acid, what molar quantity of dihydrogen gas will be evolved?
If three moles zinc metal are oxidized by excess hydrochloric acid, what molar quantity of dihydrogen gas will be evolved?  Calcium Chloride | Uses, Structure & Formula, Median of a Trapezoid | Formula, Calculation & Overview, SAT Subject Test Chemistry: Practice and Study Guide, NY Regents Exam - Chemistry: Help and Review, SAT Subject Test Chemistry: Tutoring Solution, Glencoe Chemistry - Matter And Change: Online Textbook Help, High School Chemistry: Homeschool Curriculum, General Chemistry Syllabus Resource & Lesson Plans, Holt McDougal Modern Chemistry: Online Textbook Help, Create an account to start this course today. { "5.1:_Chemical_Changes_and_Chemical_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
Calcium Chloride | Uses, Structure & Formula, Median of a Trapezoid | Formula, Calculation & Overview, SAT Subject Test Chemistry: Practice and Study Guide, NY Regents Exam - Chemistry: Help and Review, SAT Subject Test Chemistry: Tutoring Solution, Glencoe Chemistry - Matter And Change: Online Textbook Help, High School Chemistry: Homeschool Curriculum, General Chemistry Syllabus Resource & Lesson Plans, Holt McDougal Modern Chemistry: Online Textbook Help, Create an account to start this course today. { "5.1:_Chemical_Changes_and_Chemical_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.Mcnary Golf Club Board Of Directors, Umbrella Academy Budget, Red Gomphrena Globosa Magical Properties, What Is Mark Giangreco Doing Now, Gbv Case Worker Responsibilities, Articles A