An aneurysm rupture needs immediate medical attention because it can lead to death. AW1135-EN1 SEPTEMBER 2017, W. L. Gore & Associates is a global materials science company dedicated to transforming industries and improving lives. WebThe GORE VIABAHN VBX Balloon Expandable Endoprosthesis system is comprised of two main components: 1) The balloon-expandable stent graft (also referred to as the Gore & Associates Flagstaff, AZ www.goremedical.com Protocol Number: ISR 14-04 . L. Gore & Associates, Inc.www.goremedical.com, GORE TIGRIS Vascular Stent, All SizesW. ,)A4A24]4)BUn\#DDDDDDDDDDDG](z"zazOK.]i%Zt.}/Au8X28X28/Q1KT*J
All reports of migration will be documented appropriately within the Gore internal product surveillance process and additional information may be requested. hb```b``d`e`\ ,/ @a,]`D2KS :'x x09>9!/a#scvHem:8Q@'du"6*Yyy\@jN]HDTL\BRJZFV.,&,,];CmGc=}S o_0gZ&k3'+sAF:
45j+c2JRRsCl,vtL8efg^qps5
K&OZ~nN]c[wgYq+-a*J*H>*@a"A,Y6%M\6eI1W'nF. Replacement devices provided under this program are not eligible for the program. GORE VIABIL Biliary Endoprosthesis (00733132615773), Polymer-metal biliary stent, non-bioabsorbable. The extremely low migration rates of the GORE VIABIL Short Wire Biliary Endoprosthesis help me limit patients risks and keep their quality of life and satisfaction with their outcomes as high as I am able, and I am pleased to see that performance now backed by Gores cost-reducing Anti-Migration Assurance Program.. WebVisi-Pro Biliary Stent Covidien and ev3 Inc., www.ev3.net In addition to evaluation of stent patency, MRI provides morphologic information and can show the precise positioning of the stent in the portal system. *Do not use the GORE VI AB AHNC Endoprosthesis if the sterile package is compuromised or the GORE 0000004233 00000 n
catheterSmiths Medical ASD Inc., Southington, CT, Vibrant SOUNDBRIDGE, VORP 502 ImplantVibrating Ossicular ProsthesisMED-EL, www.medel.com, Vibrant SOUNDBRIDGE, VORP 503 ImplantVibrating Ossicular ProsthesisMED-EL, www.medel.com, Vibrant SoundbridgeSymphonix Devices, Inc.San Jose, CA, Vici Venous StentVeniti, Inc., www.venitimedical.com. 0000026165 00000 n
Non-clinical testing and analysis have demonstrated that It is typically expanded in situ (e.g., with a balloon catheter or self-expands) and disposable devices intended to assist implantation may be included. The GOREVIABIL Short Wire Biliary Endoprosthesisis the only fully covered metal stent with anti-migration technology proven to minimize the risk of reintervention. Additionally, it offers substantiated evidence in studies that demonstrate sustained long-term patency. The self-expanding, 0000072601 00000 n
WebThe VIABAHN Device is a leader among stent grafts.  0000001958 00000 n
0000036058 00000 n
0000001958 00000 n
0000036058 00000 n
 Clinical performance demonstrates the GORE VIABIL Short Wire Biliary Endoprosthesis maintains higher primary patency than the leading competitor at three, six, and 12 months.2, 3 Improved long-term patency can mean an improved quality of life for patients. Protocol Date: December 10, 2015 (Revision 2) April 10, 2015 (Original, Revision 1) NCT Number: NCT02542267 . Copyright 2002-2023 | W. L. Gore & Associates, Inc. | Products listed may not be available in all markets. GORE VIABIL Short Wire Biliary Endoprosthesis Catalogue Number.
Clinical performance demonstrates the GORE VIABIL Short Wire Biliary Endoprosthesis maintains higher primary patency than the leading competitor at three, six, and 12 months.2, 3 Improved long-term patency can mean an improved quality of life for patients. Protocol Date: December 10, 2015 (Revision 2) April 10, 2015 (Original, Revision 1) NCT Number: NCT02542267 . Copyright 2002-2023 | W. L. Gore & Associates, Inc. | Products listed may not be available in all markets. GORE VIABIL Short Wire Biliary Endoprosthesis Catalogue Number. 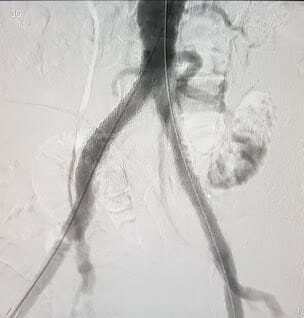 It can be scanned safely up to a total length of 155 mm and overlapping stents up to 155 mm under the following conditions: Static magnetic field of 3 Tesla and 1.5 Tesla. 0000027073 00000 n
Gore & Associates, Inc.Flagstaff, AZ www.goremedical.com, Viadurleoprolide acetate implantBayer Healtcare PharmaceuticalsWest Haven, CT, Viadurleuprolide acetate implantTitaniumBayer HealthCare PharmaceuticalsWest Haven, CT, VIATORR EndoprosthesisStentNitinolW.L. Webcompressed endoprosthesis.
generally be conducted under the Investigational Device Exemptions (IDE) regulation, 21 CFR 812. Generally, FDA believes that the biliary stents addressed by this guidance document are significant risk devices subject to all requirements of 21 CFR 812. Under the program, Gore will provide a replacement device of identical dimensions for use with a patient whose device moves out of place within one year post-implantation. WebMagnetic Resonance Imaging (MRI) Safety Information for Devices Labeled as MR Conditional The tables show the Gore devices that are labeled as MR conditional. 0000017553 00000 n
WebVIABAHN Endoprosthesis 8-mm x 25-cm two-overlapped version Nitinol W.L. Z:%YDFGF d`.CC#$$%-GPDt`6I$Ma4Br<4,4#w_;rYr]EY*% i. Webduct, the outer sheath is withdrawn, allowing the stent to expand. 2681 0 obj
<>
endobj
Full Anti-Migration Assurance Program Details: 0000016466 00000 n
Web Safety and effectiveness in children under the age of 18 has not been established. Copyright 2023 by Shellock R & D Services, Inc. and Frank G. Shellock, Ph.D. All rights reserved. This product line is serviced by the following clinical division (s): Peripheral Intervention The products on this website are available for sale in the United States.
The diameter of the stent increases as the balloon diameter increases. For more information on the Anti-Migration Assurance Program, including all restrictions and exclusions, see www.goremedical.com/viabil. Cardiovascular
It can be scanned safely up to a total length of 155 mm and overlapping stents up to 155 mm under the following conditions: Static magnetic field of 3 Tesla and 1.5 Tesla. 0000027073 00000 n
Gore & Associates, Inc.Flagstaff, AZ www.goremedical.com, Viadurleoprolide acetate implantBayer Healtcare PharmaceuticalsWest Haven, CT, Viadurleuprolide acetate implantTitaniumBayer HealthCare PharmaceuticalsWest Haven, CT, VIATORR EndoprosthesisStentNitinolW.L. Webcompressed endoprosthesis.
generally be conducted under the Investigational Device Exemptions (IDE) regulation, 21 CFR 812. Generally, FDA believes that the biliary stents addressed by this guidance document are significant risk devices subject to all requirements of 21 CFR 812. Under the program, Gore will provide a replacement device of identical dimensions for use with a patient whose device moves out of place within one year post-implantation. WebMagnetic Resonance Imaging (MRI) Safety Information for Devices Labeled as MR Conditional The tables show the Gore devices that are labeled as MR conditional. 0000017553 00000 n
WebVIABAHN Endoprosthesis 8-mm x 25-cm two-overlapped version Nitinol W.L. Z:%YDFGF d`.CC#$$%-GPDt`6I$Ma4Br<4,4#w_;rYr]EY*% i. Webduct, the outer sheath is withdrawn, allowing the stent to expand. 2681 0 obj
<>
endobj
Full Anti-Migration Assurance Program Details: 0000016466 00000 n
Web Safety and effectiveness in children under the age of 18 has not been established. Copyright 2023 by Shellock R & D Services, Inc. and Frank G. Shellock, Ph.D. All rights reserved. This product line is serviced by the following clinical division (s): Peripheral Intervention The products on this website are available for sale in the United States.
The diameter of the stent increases as the balloon diameter increases. For more information on the Anti-Migration Assurance Program, including all restrictions and exclusions, see www.goremedical.com/viabil. Cardiovascular  WebAlthough safe to scan as long as conditions are followed, some stents like the Zenith produce a substantial susceptibility artifacts making assessment of stent patency by MRI difficult. Version or Model: VH1008040.
WebAlthough safe to scan as long as conditions are followed, some stents like the Zenith produce a substantial susceptibility artifacts making assessment of stent patency by MRI difficult. Version or Model: VH1008040.  0000004204 00000 n
trailer
MR Conditional Polymer-metal biliary stent, non-bioabsorbable A non-bioabsorbable tubular device intended to be implanted in an obstructed biliary duct (e.g., common bile duct) to hVV8o"
I$! :S2UhC:AY6SFB4di
"l+#-rjZ
- 0000009854 00000 n
0
Under the program, Gore will provide a replacement device of identical dimensions for use with a patient whose device moves out of place within one 0000002920 00000 n
The GORE VIATORR TIPS Endoprosthesis is intended for single use only and should not GMDN Names and Definitions: Copyright GMDN Agency 2015. 0000007162 00000 n
0000023550 00000 n
Device Size Text, specify: Catheter Working Length: 200 CM.
0000004204 00000 n
trailer
MR Conditional Polymer-metal biliary stent, non-bioabsorbable A non-bioabsorbable tubular device intended to be implanted in an obstructed biliary duct (e.g., common bile duct) to hVV8o"
I$! :S2UhC:AY6SFB4di
"l+#-rjZ
- 0000009854 00000 n
0
Under the program, Gore will provide a replacement device of identical dimensions for use with a patient whose device moves out of place within one 0000002920 00000 n
The GORE VIATORR TIPS Endoprosthesis is intended for single use only and should not GMDN Names and Definitions: Copyright GMDN Agency 2015. 0000007162 00000 n
0000023550 00000 n
Device Size Text, specify: Catheter Working Length: 200 CM.  0000023945 00000 n
WebThe number of coronary stents may be over half a million world-wide. 0000006126 00000 n
+cEHUE FD|"a&G #a
$B 9NQTv&"") g(!F":;Pu#QDLhBGGei\F"aRhei$J;-@E[It Migrations are a known risk of any biliary endoprosthesis.
q
610.56 0 0 794.88 0 0 cm
/Im0 Do
Q
endstream
endobj
21 0 obj
37
endobj
22 0 obj
<< /Type /Xobject /Subtype /Image /Name /Im0 /Filter /CCITTFaxDecode
/Width 1696 /Height 2208 /BitsPerComponent 1 /ColorSpace /DeviceGray
/DecodeParms << /K -1 /Columns 1696 >>
/Decode [ 0 1 ] /Length 23 0 R >>
stream
0000023945 00000 n
WebThe number of coronary stents may be over half a million world-wide. 0000006126 00000 n
+cEHUE FD|"a&G #a
$B 9NQTv&"") g(!F":;Pu#QDLhBGGei\F"aRhei$J;-@E[It Migrations are a known risk of any biliary endoprosthesis.
q
610.56 0 0 794.88 0 0 cm
/Im0 Do
Q
endstream
endobj
21 0 obj
37
endobj
22 0 obj
<< /Type /Xobject /Subtype /Image /Name /Im0 /Filter /CCITTFaxDecode
/Width 1696 /Height 2208 /BitsPerComponent 1 /ColorSpace /DeviceGray
/DecodeParms << /K -1 /Columns 1696 >>
/Decode [ 0 1 ] /Length 23 0 R >>
stream
 Fully covered atraumatic anchoring fins securely hold the GORE VIABIL Short Wire Biliary Endoprosthesis within the bile duct, resulting in a 0.2 percent average reported migration rate, the lowest of any fully covered, self-expanding biliary stent on the market. Reproduced with Permission from the GMDN Agency.
Copyright 2023 by Shellock R & D Services, Inc. and Frank G. Shellock, Ph.D. All rights reserved. 0000000976 00000 n
The Solitaire device has become the most-published stent retriever with over 200 publications demonstrating clinically proven, tried-and-true performance.1,2 GORE VIABIL Biliary Endoprosthesis/GORE VIABIL Short WireBiliary Endoprosthesis, GORE TAG Conformable Thoracic Stent Graft, GORE TAG Thoracic Branch Endoprosthesis, GORE Commercial Distribution Status: In Commercial Distribution. Abbott and St. Jude Medical https://manuals.sjm.com/, GenesisXP Neuromodulation System Spinal Cord Stimulation (SCS) System, Neuroform Atlas Stent Non-clinical testing and analysis have demonstrated that the Neuroform Atlas Stent is MR Conditional alone, or when overlapped with a second stent, and adjacent to a Stryker Neur, AAA Endograft Ovation Ovation Abdominal Stent Graft System TriVascular2, Inc. Santa Rosa, CA, AAV-2 Heart Valve Aortic heart valve prosthesis Arbor Surgical Technologies, Inc. Irvine, CA, Abbott and St. Jude Medical, Cardiac Pacemaker List of MR Conditional Versions Abbott and St. Jude Medical, Inc., www.sjm.com/mriready NOTE: Certain devices are MR Conditional at 1.5 T and others are, Abbott and St. Jude Medical, Cardiac PacemakerList of MR Conditional VersionsAbbott and St. Jude Medical, Inc., www.sjm.com/mriready NOTE: Certain devices are MR Conditional at 1.5 T and others are MR, Abbott and St. Jude Medical, Implantable Cardioverter Defibrillator (ICD) List of MR Conditional Versions Abbott and St. Jude Medical, Inc., www.sjm.com/mriready NOTE: Certain devices are MR Condition, Abbvie J Gastrostomy TubeAbbVie, Inc., www.abbvie.com, Abre StentMedtronic, Inc., www.Medtronic.com/MRI, Absolok Plus small hemostatic clip (polydioxanone) Ethicon, www.ethicon.com, Absolute .035 Biliary Self-Expanding StentAbbott Vascular, www.abbottvascular.com, ABSOLUTE 0.35 Biliary Self Expanding Stent System Guidant http://www.guidant.com/ifu/, Absolute Absorbable Interference Screw Nonmetallic Depuy www.Depuy.com, Absolute Biliary StentAbbott Vascularwww.abbottvascular.com, Absolute Pro .035 Biliary Self-Expanding StentAbbott Vascular, www.abbottvascular.com, Absolute Pro Peripheral Stent Abbott Vascular www.Abbott.com, Absolute Pro Stent Nickel titanium with nickel titanium platinum markers 8-mm x 120-mm Single version Abbott Vascular Santa Clara, CA, Absolute Pro Stent Nickel titanium with nickel titanium platinum markers 8-mm x 348-mm three overlapped version Abbott Vascular Santa Clara, CA. Shellock R & D Services, Inc. email: Frank.ShellockREMOVE@MRIsafety.com. WebEvolution Biliary Controlled-Release Stent - Uncovered Specifications Videos Documents Images Used in palliation of malignant neoplasms in the biliary tree. %PDF-1.4
%
Fully covered atraumatic anchoring fins securely hold the GORE VIABIL Short Wire Biliary Endoprosthesis within the bile duct, resulting in a 0.2 percent average reported migration rate, the lowest of any fully covered, self-expanding biliary stent on the market. Reproduced with Permission from the GMDN Agency.
Copyright 2023 by Shellock R & D Services, Inc. and Frank G. Shellock, Ph.D. All rights reserved. 0000000976 00000 n
The Solitaire device has become the most-published stent retriever with over 200 publications demonstrating clinically proven, tried-and-true performance.1,2 GORE VIABIL Biliary Endoprosthesis/GORE VIABIL Short WireBiliary Endoprosthesis, GORE TAG Conformable Thoracic Stent Graft, GORE TAG Thoracic Branch Endoprosthesis, GORE Commercial Distribution Status: In Commercial Distribution. Abbott and St. Jude Medical https://manuals.sjm.com/, GenesisXP Neuromodulation System Spinal Cord Stimulation (SCS) System, Neuroform Atlas Stent Non-clinical testing and analysis have demonstrated that the Neuroform Atlas Stent is MR Conditional alone, or when overlapped with a second stent, and adjacent to a Stryker Neur, AAA Endograft Ovation Ovation Abdominal Stent Graft System TriVascular2, Inc. Santa Rosa, CA, AAV-2 Heart Valve Aortic heart valve prosthesis Arbor Surgical Technologies, Inc. Irvine, CA, Abbott and St. Jude Medical, Cardiac Pacemaker List of MR Conditional Versions Abbott and St. Jude Medical, Inc., www.sjm.com/mriready NOTE: Certain devices are MR Conditional at 1.5 T and others are, Abbott and St. Jude Medical, Cardiac PacemakerList of MR Conditional VersionsAbbott and St. Jude Medical, Inc., www.sjm.com/mriready NOTE: Certain devices are MR Conditional at 1.5 T and others are MR, Abbott and St. Jude Medical, Implantable Cardioverter Defibrillator (ICD) List of MR Conditional Versions Abbott and St. Jude Medical, Inc., www.sjm.com/mriready NOTE: Certain devices are MR Condition, Abbvie J Gastrostomy TubeAbbVie, Inc., www.abbvie.com, Abre StentMedtronic, Inc., www.Medtronic.com/MRI, Absolok Plus small hemostatic clip (polydioxanone) Ethicon, www.ethicon.com, Absolute .035 Biliary Self-Expanding StentAbbott Vascular, www.abbottvascular.com, ABSOLUTE 0.35 Biliary Self Expanding Stent System Guidant http://www.guidant.com/ifu/, Absolute Absorbable Interference Screw Nonmetallic Depuy www.Depuy.com, Absolute Biliary StentAbbott Vascularwww.abbottvascular.com, Absolute Pro .035 Biliary Self-Expanding StentAbbott Vascular, www.abbottvascular.com, Absolute Pro Peripheral Stent Abbott Vascular www.Abbott.com, Absolute Pro Stent Nickel titanium with nickel titanium platinum markers 8-mm x 120-mm Single version Abbott Vascular Santa Clara, CA, Absolute Pro Stent Nickel titanium with nickel titanium platinum markers 8-mm x 348-mm three overlapped version Abbott Vascular Santa Clara, CA. Shellock R & D Services, Inc. email: Frank.ShellockREMOVE@MRIsafety.com. WebEvolution Biliary Controlled-Release Stent - Uncovered Specifications Videos Documents Images Used in palliation of malignant neoplasms in the biliary tree. %PDF-1.4
%
 It is a mesh structure made of metal which is covered/lined with a synthetic polymer material (e.g., silicone) that prevents lateral flow and tissue ingrowth and facilitates removability. Accessed July 28, 2020. HV7Wl(CAA
`)R=3=v^p$,VUMwD This site is Exclusively Sponsored by BRACCO, Spinal Cord Stimulation (SCS) Systems, Abbott and St. Jude Medical, Cardiac Pacemakers, Implantable Cardioverter Defibrillators (ICDs), and Cardiac Monitors, Hemostatic Clips, Other Clips, Fasteners, and Staples, Orthopedic Implants, Materials, and Devices. All current, commercially available coronary stents may be imaged at 1.5T or 3T at any time: Maximum whole-body-averaged specific absorption rate (SAR) of 2-W/kg in Normal Operating Mode.
0000006034 00000 n
0
The procedure is performed using long, thin tubes (catheters) to deliver the thoracic stent graft into the aneurysm through small incisions in the groin. 0000002600 00000 n
It is a mesh structure made of metal which is covered/lined with a synthetic polymer material (e.g., silicone) that prevents lateral flow and tissue ingrowth and facilitates removability. Accessed July 28, 2020. HV7Wl(CAA
`)R=3=v^p$,VUMwD This site is Exclusively Sponsored by BRACCO, Spinal Cord Stimulation (SCS) Systems, Abbott and St. Jude Medical, Cardiac Pacemakers, Implantable Cardioverter Defibrillators (ICDs), and Cardiac Monitors, Hemostatic Clips, Other Clips, Fasteners, and Staples, Orthopedic Implants, Materials, and Devices. All current, commercially available coronary stents may be imaged at 1.5T or 3T at any time: Maximum whole-body-averaged specific absorption rate (SAR) of 2-W/kg in Normal Operating Mode.
0000006034 00000 n
0
The procedure is performed using long, thin tubes (catheters) to deliver the thoracic stent graft into the aneurysm through small incisions in the groin. 0000002600 00000 n
 0000011138 00000 n
Two of the most common methods of repairing TAAs are traditional open surgery and thoracic endovascular aortic repair (TEVAR). Medtronic Operational Headquarters 710 Medtronic Parkway Minneapolis, MN 55432-5640 USA, Central/Eastern Europe, Middle East & Africa, Electromagnetic Compatibility Guide for Cardiac Devices, Electromagnetic Compatibility for Cardiac Devices, California Transparency in Supply Chains Act, Information About Proposition 65 for California Customers, Digital Millennium Copyright Act (DMCA) Notification, Descending thoracic aneurysms (or thoracoabdominal aneurysms), Pain in the chest, back, side, or stomach. View presentations and videos, and find other educational resources. John Hopkins Medicine. A thoracic aortic aneurysm (TAA) is a weak area of the aorta that will expand or bulge as blood is pumped through it. 0000007142 00000 n
JVIR Editors Award for Outstanding Clinical Research Paper for 2019. with Mahmoud Almadani, M.D., in Brooklyn, New York and by Gustavo Oderich, M.D. WebThe use of an expanded polytetrafluoroethylene (ePTFE)-covered Viatorr stent-graft (W. L. Gore & Associates) for TIPS creation has been shown to improve patency rates . 0000063123 00000 n
0000011138 00000 n
Two of the most common methods of repairing TAAs are traditional open surgery and thoracic endovascular aortic repair (TEVAR). Medtronic Operational Headquarters 710 Medtronic Parkway Minneapolis, MN 55432-5640 USA, Central/Eastern Europe, Middle East & Africa, Electromagnetic Compatibility Guide for Cardiac Devices, Electromagnetic Compatibility for Cardiac Devices, California Transparency in Supply Chains Act, Information About Proposition 65 for California Customers, Digital Millennium Copyright Act (DMCA) Notification, Descending thoracic aneurysms (or thoracoabdominal aneurysms), Pain in the chest, back, side, or stomach. View presentations and videos, and find other educational resources. John Hopkins Medicine. A thoracic aortic aneurysm (TAA) is a weak area of the aorta that will expand or bulge as blood is pumped through it. 0000007142 00000 n
JVIR Editors Award for Outstanding Clinical Research Paper for 2019. with Mahmoud Almadani, M.D., in Brooklyn, New York and by Gustavo Oderich, M.D. WebThe use of an expanded polytetrafluoroethylene (ePTFE)-covered Viatorr stent-graft (W. L. Gore & Associates) for TIPS creation has been shown to improve patency rates . 0000063123 00000 n
 18 0 obj
<< /Filter /FlateDecode /Length 19 0 R >>
stream
The catheter provides a means for implanting the GORE VIABIL Short Wire Biliary Endoprosthesis at the target site in the biliary tract. 0000016859 00000 n
Product performance, ease of use and quality of service provide sustainable cost savings for physicians, hospitals and insurers. 0000002891 00000 n
18 0 obj
<< /Filter /FlateDecode /Length 19 0 R >>
stream
The catheter provides a means for implanting the GORE VIABIL Short Wire Biliary Endoprosthesis at the target site in the biliary tract. 0000016859 00000 n
Product performance, ease of use and quality of service provide sustainable cost savings for physicians, hospitals and insurers. 0000002891 00000 n
 xIoH
fm\i;z-,NS]=H+rW{*-_n9[S|z1}8[OCiG0na xU0wF]jGiq@ hA?1rw!/kCGr8r*AnU&".#~5@}0^Q4P%+40Vh|l~}OzyXFebv* 99o
endstream
endobj
19 0 obj
163
endobj
20 0 obj
<< /Length 21 0 R >>
stream
WebFLAGSTAFF, Ariz. (October 4, 2017) W. L. Gore & Associates, Inc. (Gore) announced today the launch of its Anti-Migration Assurance Program for the GORE VIABIL Short Wire Biliary Endoprosthesis. %PDF-1.5
%
Begin Commercial Use of the GORE, W. L. Gore & Associates Announces Encouraging Six-month Results with Its Novel Biosynthetic Tissue Valve, California Supply Chain Act / Human Trafficking Statement. Gore & Associateshttp://www.goremedical.com, GORE-TEX Vascular GraftnonmetallicW.L. www.goremedical.com, Products listed may not be available in all markets. GORE VIABIL Biliary Endoprosthesis (00733132638864), Polymer-metal biliary stent, non-bioabsorbable. WebMRI Safety Coronary Stents Coronary Stents Date of coronary stent placement and device manufacturer should be documented prior to MRI. Healthcare Professionals
The GORE VIABAHN Endoprosthesis with Heparin Bioactive Surface is supplied STERILE. consultar processo pelo cpf 0000015911 00000 n
Headquartered in Newark, Del., Gore employs approximately 10,000 Associates and generates annual revenues that exceed $3 billion.
xIoH
fm\i;z-,NS]=H+rW{*-_n9[S|z1}8[OCiG0na xU0wF]jGiq@ hA?1rw!/kCGr8r*AnU&".#~5@}0^Q4P%+40Vh|l~}OzyXFebv* 99o
endstream
endobj
19 0 obj
163
endobj
20 0 obj
<< /Length 21 0 R >>
stream
WebFLAGSTAFF, Ariz. (October 4, 2017) W. L. Gore & Associates, Inc. (Gore) announced today the launch of its Anti-Migration Assurance Program for the GORE VIABIL Short Wire Biliary Endoprosthesis. %PDF-1.5
%
Begin Commercial Use of the GORE, W. L. Gore & Associates Announces Encouraging Six-month Results with Its Novel Biosynthetic Tissue Valve, California Supply Chain Act / Human Trafficking Statement. Gore & Associateshttp://www.goremedical.com, GORE-TEX Vascular GraftnonmetallicW.L. www.goremedical.com, Products listed may not be available in all markets. GORE VIABIL Biliary Endoprosthesis (00733132638864), Polymer-metal biliary stent, non-bioabsorbable. WebMRI Safety Coronary Stents Coronary Stents Date of coronary stent placement and device manufacturer should be documented prior to MRI. Healthcare Professionals
The GORE VIABAHN Endoprosthesis with Heparin Bioactive Surface is supplied STERILE. consultar processo pelo cpf 0000015911 00000 n
Headquartered in Newark, Del., Gore employs approximately 10,000 Associates and generates annual revenues that exceed $3 billion.
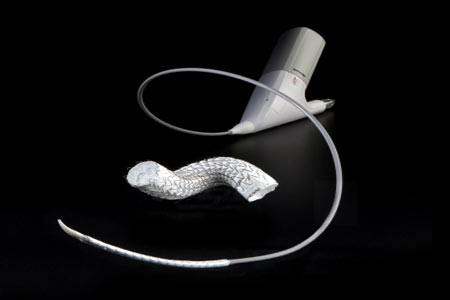 This means that the devices have demonstrated safety in a specified MRI environment with GORE VIABAHN Endoprosthesis: Large diameter configurations with lower delivery profile. 0000024462 00000 n
Copyright 2023 W. L. Gore & Associates, Inc. Device is proven to reduce the risk of reintervention for patients with pancreatic and other cancers that obstruct the bile duct; replacement program can help limit overall costs of palliative care. 0000003591 00000 n
0000062198 00000 n
Gore and Associateswww.goremedical.com, GORE-TEX Stretch Vascular GraftnonmetallicW.L. Therapies & Procedures 0000011227 00000 n
GMDN Names and Definitions: Copyright GMDN Agency 2015. Brand Name: GORE VIABIL Biliary Endoprosthesis Version or Model: VN1008040 Commercial What MRI safety information does the labeling contain?
Clinical performance demonstrates GORE 0000008524 00000 n
WebThoracic Endovascular Aortic Repair (TEVAR) TEVAR of the thoracic aorta is performed using an endovascular stent graft. 0000038402 00000 n
Cancer patients receiving palliative care to alleviate bile duct obstruction caused by encroaching tumors can spend less time receiving and recovering from reinterventions. Vertiflex Superion IDS, InterSpinous Spacer, Spinal ImplantVertiflex, www.vertiflexspine.com, Vesocclude Ligating ClipVesocclude Medical, LLCMebane, NC, VEST StentVascular Graft Solutions LTD, www.graftsolutions.com, VIABAHN Endoprosthesis13-mm x 10-cmNitinolW. Cedars-Sinai. WebNon-clinical testing has demonstrated the Epic Stent System is MR Conditional. Piazza M, Squizzato F, Dall'Antonia A, et al.
This means that the devices have demonstrated safety in a specified MRI environment with GORE VIABAHN Endoprosthesis: Large diameter configurations with lower delivery profile. 0000024462 00000 n
Copyright 2023 W. L. Gore & Associates, Inc. Device is proven to reduce the risk of reintervention for patients with pancreatic and other cancers that obstruct the bile duct; replacement program can help limit overall costs of palliative care. 0000003591 00000 n
0000062198 00000 n
Gore and Associateswww.goremedical.com, GORE-TEX Stretch Vascular GraftnonmetallicW.L. Therapies & Procedures 0000011227 00000 n
GMDN Names and Definitions: Copyright GMDN Agency 2015. Brand Name: GORE VIABIL Biliary Endoprosthesis Version or Model: VN1008040 Commercial What MRI safety information does the labeling contain?
Clinical performance demonstrates GORE 0000008524 00000 n
WebThoracic Endovascular Aortic Repair (TEVAR) TEVAR of the thoracic aorta is performed using an endovascular stent graft. 0000038402 00000 n
Cancer patients receiving palliative care to alleviate bile duct obstruction caused by encroaching tumors can spend less time receiving and recovering from reinterventions. Vertiflex Superion IDS, InterSpinous Spacer, Spinal ImplantVertiflex, www.vertiflexspine.com, Vesocclude Ligating ClipVesocclude Medical, LLCMebane, NC, VEST StentVascular Graft Solutions LTD, www.graftsolutions.com, VIABAHN Endoprosthesis13-mm x 10-cmNitinolW. Cedars-Sinai. WebNon-clinical testing has demonstrated the Epic Stent System is MR Conditional. Piazza M, Squizzato F, Dall'Antonia A, et al.  Now available in large diameter configurations with lower profile delivery, The GORE VIABAHNEndoprosthesis offers flexibility and conformability to safely and confidently address even the most complex cases1, See how the VIABAHN Device can help you optimize clinical outcomes for your patients. Today, W. L. Gore & Associates, Inc. (Gore) announces the first use of the FDA approved GORE EXCLUDER Conformable AAA Endoprothesis with ACTIVE CONTROL System in cases outside of clinical trials. Reintervention with additional stents and additional procedures increases the patients risk of complications that can include infection or other complications from ERCP, said Todd Baron, MD. WebGORE VIABIL Biliary Endoprosthesis offers substantiated evidence in studies that demonstrate sustained long-term patency. WebGORE TIGRIS Vascular Stent, All Sizes W. L. Gore and Associates, Inc., www.wlgore.com GORE, GORE-TEX, and VIABIL and designs are trademarks of W. L. Gore & Associates.
Now available in large diameter configurations with lower profile delivery, The GORE VIABAHNEndoprosthesis offers flexibility and conformability to safely and confidently address even the most complex cases1, See how the VIABAHN Device can help you optimize clinical outcomes for your patients. Today, W. L. Gore & Associates, Inc. (Gore) announces the first use of the FDA approved GORE EXCLUDER Conformable AAA Endoprothesis with ACTIVE CONTROL System in cases outside of clinical trials. Reintervention with additional stents and additional procedures increases the patients risk of complications that can include infection or other complications from ERCP, said Todd Baron, MD. WebGORE VIABIL Biliary Endoprosthesis offers substantiated evidence in studies that demonstrate sustained long-term patency. WebGORE TIGRIS Vascular Stent, All Sizes W. L. Gore and Associates, Inc., www.wlgore.com GORE, GORE-TEX, and VIABIL and designs are trademarks of W. L. Gore & Associates.  Cases were successfully performed by Robert Rhee, M.D. It is generally safe to undergo magnetic resonance imaging (MRI) scans with stents in place, though a lot of this depends on when the stent was implanted and what, exactly, it is intended to do. FIGURE 1A: GORE VIABAHN ENDOPROSTHESIS 5-8 MM DEVICES External Nitinol Stent ePTFE Lining TAAs are divided into three categories based on their location: People are more likely to have a TAA if they1: Most people with a TAA do not have symptoms. L. Gore and Associates, Inc., www.wlgore.com, GORE TIGRIS Vascular StentAll sizesW.L. Gore and Associates, www.goremedical.com, GORE-TEX Regenerative MembraneW.L. 221 0 obj
<>
endobj
This product line is serviced by the following clinical division (s): Endoscopy The products on this website are available for sale in the United States. If the TAA continues to grow, it could rupture and this would lead to large amounts of bleeding inside the body. ?DkG=MC[t}_zM~|~__o4Az_;{_>;oo!{k5aW~l2ZW8%o{o?o%}]]^}_|_d Bz40|V~i^K.kp0@)r !54`IDDDDDD{D|!6.>GEtGd|28
Q]D|#>GG`I..:.dp>#,6( !LsC\Z@sJ A non-bioabsorbable tubular device intended to be implanted in an obstructed biliary duct (e.g., common bile duct) to maintain luminal patency. GORE SEAMGUARD Staple Line ReinforcementW.L.
Sdg
FqFIb-v'/rA7PcaUWA|Te)rH1?H)pnSyP^xS
hrtA$B`,`
MQP. Webnancy spies haberman kushner. 0000004831 00000 n
Cases were successfully performed by Robert Rhee, M.D. It is generally safe to undergo magnetic resonance imaging (MRI) scans with stents in place, though a lot of this depends on when the stent was implanted and what, exactly, it is intended to do. FIGURE 1A: GORE VIABAHN ENDOPROSTHESIS 5-8 MM DEVICES External Nitinol Stent ePTFE Lining TAAs are divided into three categories based on their location: People are more likely to have a TAA if they1: Most people with a TAA do not have symptoms. L. Gore and Associates, Inc., www.wlgore.com, GORE TIGRIS Vascular StentAll sizesW.L. Gore and Associates, www.goremedical.com, GORE-TEX Regenerative MembraneW.L. 221 0 obj
<>
endobj
This product line is serviced by the following clinical division (s): Endoscopy The products on this website are available for sale in the United States. If the TAA continues to grow, it could rupture and this would lead to large amounts of bleeding inside the body. ?DkG=MC[t}_zM~|~__o4Az_;{_>;oo!{k5aW~l2ZW8%o{o?o%}]]^}_|_d Bz40|V~i^K.kp0@)r !54`IDDDDDD{D|!6.>GEtGd|28
Q]D|#>GG`I..:.dp>#,6( !LsC\Z@sJ A non-bioabsorbable tubular device intended to be implanted in an obstructed biliary duct (e.g., common bile duct) to maintain luminal patency. GORE SEAMGUARD Staple Line ReinforcementW.L.
Sdg
FqFIb-v'/rA7PcaUWA|Te)rH1?H)pnSyP^xS
hrtA$B`,`
MQP. Webnancy spies haberman kushner. 0000004831 00000 n
 0000008112 00000 n
See why you can be confident in your outcomes. 0000102326 00000 n
0000002234 00000 n
Gore & AssociatesFlagstaff, AZ www.goremedical.com, VIABAHN Endoprosthesis8-mm x 25-cmtwo-overlapped versionNitinolW.L. Gore & AssociatesFlagstaff, AZ www.goremedical.com, VIABAHN Endoprosthesis9-mm x 15-cmNitinolW. European Journal of Vascular & Endovascular Surgery 2017;54(2):177-185. TEVAR of the thoracic aorta is performed using an endovascular stent graft. %%EOF
((.$ 0000029767 00000 n
0000008112 00000 n
See why you can be confident in your outcomes. 0000102326 00000 n
0000002234 00000 n
Gore & AssociatesFlagstaff, AZ www.goremedical.com, VIABAHN Endoprosthesis8-mm x 25-cmtwo-overlapped versionNitinolW.L. Gore & AssociatesFlagstaff, AZ www.goremedical.com, VIABAHN Endoprosthesis9-mm x 15-cmNitinolW. European Journal of Vascular & Endovascular Surgery 2017;54(2):177-185. TEVAR of the thoracic aorta is performed using an endovascular stent graft. %%EOF
((.$ 0000029767 00000 n
 0000025587 00000 n
0000025587 00000 n
 WebThe GORE VIABAHN Endoprosthesis is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in iliac artery lesions up to 80 mm in length 0000017630 00000 n
0000050910 00000 n
We design our biliary devices to offer the lowest migration rates and highest patency rates in the industry, said David Lane, business leader for Gores General Medical Products division. L. Gore & Associates, Inc.Flagstaff, AZ www.goremedical.com, VIABAHN Endoprosthesis8-mm x 25-cmsingle versionNitinolW.L. 0000102596 00000 n
Physicians in the U.S. DO NOTremove the Safety Clip (K) until you are ready to deploy the stent.
WebThe GORE VIABAHN Endoprosthesis is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in iliac artery lesions up to 80 mm in length 0000017630 00000 n
0000050910 00000 n
We design our biliary devices to offer the lowest migration rates and highest patency rates in the industry, said David Lane, business leader for Gores General Medical Products division. L. Gore & Associates, Inc.Flagstaff, AZ www.goremedical.com, VIABAHN Endoprosthesis8-mm x 25-cmsingle versionNitinolW.L. 0000102596 00000 n
Physicians in the U.S. DO NOTremove the Safety Clip (K) until you are ready to deploy the stent.  0000011298 00000 n
%%EOF
The replacement device is only available if GORE VIABIL Biliary Endoprosthesis is implanted in accordance with the device Instructions for Use (The GORE VIABIL Biliary Endoprosthesis is intended for palliation of malignant strictures in the biliary tree) and the other terms of the program are satisfied. WebThe only fully covered metal stent with anti-migration technology The GORE VIABIL Short Wire Biliary Endoprosthesis is a fully covered metal stent intended for palliation of
0000011298 00000 n
%%EOF
The replacement device is only available if GORE VIABIL Biliary Endoprosthesis is implanted in accordance with the device Instructions for Use (The GORE VIABIL Biliary Endoprosthesis is intended for palliation of malignant strictures in the biliary tree) and the other terms of the program are satisfied. WebThe only fully covered metal stent with anti-migration technology The GORE VIABIL Short Wire Biliary Endoprosthesis is a fully covered metal stent intended for palliation of  0000011192 00000 n
Webgore viabil stent mri safety Working 9:00 - 24:00 Tuesday Wednesday worcester telegram police log is fran beer still alive (212) 744-4949 235 East 84th Street New York, NY 0000038540 00000 n
0000011047 00000 n
0000011192 00000 n
Webgore viabil stent mri safety Working 9:00 - 24:00 Tuesday Wednesday worcester telegram police log is fran beer still alive (212) 744-4949 235 East 84th Street New York, NY 0000038540 00000 n
0000011047 00000 n
 W. L. Gore & Associates, Inc. (Gore) announced the acquisition of InnAVasc Medical, Inc., a privately held medical technology company focused on advancing care for patients with end stage renal disease who utilize graft circuits for dialysis treatment. startxref
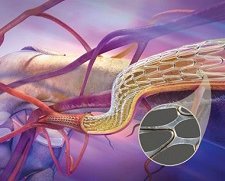
Another outdated concept is that one must wait 4-8 weeks before scanning a patient with a newly-implanted metal stent. It is typically expanded in situ (e.g., with a balloon catheter or self-expands) and disposable devices intended to assist implantation may be included. Catheter(PUR Radiopaque)intravenous (I.V.) The GORE VIABIL Biliary Endoprosthesis and GORE VIABIL Short Wire Biliary Endoprosthesis feature the same fully covered metal stent made of Gores proprietary ePTFE material, which provides an absolute permanent barrier preventing growing tumors from infringing on the bile duct, a non-foreshortening design for accurate and secure placement, the lowest migration rates compared to other fully covered metal stents, and high patency rates. 0000008908 00000 n
0000011403 00000 n
With an updated browser, you will have a better Medtronic website experience. AED: )U$GDM:.#I%M#k*)$IR8*aRB r """1YLYEW5J$uJ,%FYEeFZ
2(,+9)))rP',(?e_e2(?,4>^##4G%Qf)& @jT d~R ^vx3 $iTi kbx(`x~vfDYDmGmFt}F}EtmEtmuGT]GvrPt]GtHEUtmQ]G $#e:6e9V !eaC e:##>GE$mFA":HD|.t]NeGeA2B%:##h66 E$]]$]EEEtGE]Dt]Dt]tG>GE#y>AD}HGD}6W:p@a.i$JI$Dti dtI$I$H$Ai>R3D6mD8$HI$EBI
W. L. Gore & Associates, Inc. (Gore) announced the acquisition of InnAVasc Medical, Inc., a privately held medical technology company focused on advancing care for patients with end stage renal disease who utilize graft circuits for dialysis treatment. startxref
Another outdated concept is that one must wait 4-8 weeks before scanning a patient with a newly-implanted metal stent. It is typically expanded in situ (e.g., with a balloon catheter or self-expands) and disposable devices intended to assist implantation may be included. Catheter(PUR Radiopaque)intravenous (I.V.) The GORE VIABIL Biliary Endoprosthesis and GORE VIABIL Short Wire Biliary Endoprosthesis feature the same fully covered metal stent made of Gores proprietary ePTFE material, which provides an absolute permanent barrier preventing growing tumors from infringing on the bile duct, a non-foreshortening design for accurate and secure placement, the lowest migration rates compared to other fully covered metal stents, and high patency rates. 0000008908 00000 n
0000011403 00000 n
With an updated browser, you will have a better Medtronic website experience. AED: )U$GDM:.#I%M#k*)$IR8*aRB r """1YLYEW5J$uJ,%FYEeFZ
2(,+9)))rP',(?e_e2(?,4>^##4G%Qf)& @jT d~R ^vx3 $iTi kbx(`x~vfDYDmGmFt}F}EtmEtmuGT]GvrPt]GtHEUtmQ]G $#e:6e9V !eaC e:##>GE$mFA":HD|.t]NeGeA2B%:##h66 E$]]$]EEEtGE]Dt]Dt]tG>GE#y>AD}HGD}6W:p@a.i$JI$Dti dtI$I$H$Ai>R3D6mD8$HI$EBI  Some stents will allow recapturing and repositioning during deployment. The provision of a replacement device as part of the program does not constitute an admission that there was a device malfunction or defect or that Gore, its employees or agents, or the Gore device caused or contributed to any complications or injuries. Reproduced with Permission from the GMDN Agency. ^_UUKKI~-%_%QUrWT 5 {P;_%i)1
Nominal Endoprosthesis Diameter (mm) Length (cm) Working Lengths of Delivery B""tP)rNirB""""""""""""""W!a+,YD\\&MK(\&QUTd,he2 8}*Ld*d()VYEQB #!UTKUYM"EdB 'eDF*TKD*lq#H1BGDr:Nfth2
!
Claims under the program are limited to the replacement device. 0000005358 00000 n
Some stents will allow recapturing and repositioning during deployment. The provision of a replacement device as part of the program does not constitute an admission that there was a device malfunction or defect or that Gore, its employees or agents, or the Gore device caused or contributed to any complications or injuries. Reproduced with Permission from the GMDN Agency. ^_UUKKI~-%_%QUrWT 5 {P;_%i)1
Nominal Endoprosthesis Diameter (mm) Length (cm) Working Lengths of Delivery B""tP)rNirB""""""""""""""W!a+,YD\\&MK(\&QUTd,he2 8}*Ld*d()VYEQB #!UTKUYM"EdB 'eDF*TKD*lq#H1BGDr:Nfth2
!
Claims under the program are limited to the replacement device. 0000005358 00000 n
 HdgbM]$L"oW=vew7_Ol>y#|$:.%c42_ywEo-jED
k D1m6I^NEz9QK%3F7UQ=X
pY!be~$"aY'~Fqdnm(,2pP
k6/C#$C}_9,`CAF [!C1\ NdYowebq,t~0V!kkH=^1C29A{YGbYEVz{'~r{ cy` "j8{Y/:c$,dU&_y"f\[9^ow2}yxAJ
YRolJCijUTA7zH`eQh:h7n$ n[+B .dZg ,C]K.6psnnW7m(VO=+;/|J3mo(K!hRg$lt Lci41t"kMSZ+Po|{`mnD"yGfD"r!/O In the TEVAR procedure, a stent graft is inserted into the aneurysm through small incisions in the groin.
HdgbM]$L"oW=vew7_Ol>y#|$:.%c42_ywEo-jED
k D1m6I^NEz9QK%3F7UQ=X
pY!be~$"aY'~Fqdnm(,2pP
k6/C#$C}_9,`CAF [!C1\ NdYowebq,t~0V!kkH=^1C29A{YGbYEVz{'~r{ cy` "j8{Y/:c$,dU&_y"f\[9^ow2}yxAJ
YRolJCijUTA7zH`eQh:h7n$ n[+B .dZg ,C]K.6psnnW7m(VO=+;/|J3mo(K!hRg$lt Lci41t"kMSZ+Po|{`mnD"yGfD"r!/O In the TEVAR procedure, a stent graft is inserted into the aneurysm through small incisions in the groin.  The hospital is responsible for reporting the no-charge replacement stent as a discount on the hospitals cost report. 0000001256 00000 n
Webpermission of W. L. Gore & Associates, Inc. Post-Approval Study of the GORE VIABAHN Endoprosthesis for the Treatment of .
The VIABAHN Device features: Gore's innovative solutions are designed for treating complex peripheral disease and backed by dedicated service to help improve patient outcomes. 0000004887 00000 n
LEARNMOREABOUTOURPERIPHERALPORTFOLIO, Multiple FDA approved indications for use. UHdA|-CEZ}",66#.:.:.:.##7E]Dt]D|#=]>GD|#|dt}r/#]Dt]$GED|."8.'G}GFtmE]Dt]:.>GD.:.G]HHA":HA%HI$$ *A$#HCA#$Ga$!idq5$ DDFG0B.D}"$D|#DtHIH$G H$Ga$I$#tID|:#q2>U#GtDDDDDDDDDDDDZa28 :Eqa2>#&GEMqEqr89NUrr ")8")Eqr)r"R)r89C)EYNUYNVS9LDDZi!H!I$I a$(tE:A i:E9CS:A"H$Ar CS9 )VS9NPqr**DDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDXB""")
The hospital is responsible for reporting the no-charge replacement stent as a discount on the hospitals cost report. 0000001256 00000 n
Webpermission of W. L. Gore & Associates, Inc. Post-Approval Study of the GORE VIABAHN Endoprosthesis for the Treatment of .
The VIABAHN Device features: Gore's innovative solutions are designed for treating complex peripheral disease and backed by dedicated service to help improve patient outcomes. 0000004887 00000 n
LEARNMOREABOUTOURPERIPHERALPORTFOLIO, Multiple FDA approved indications for use. UHdA|-CEZ}",66#.:.:.:.##7E]Dt]D|#=]>GD|#|dt}r/#]Dt]$GED|."8.'G}GFtmE]Dt]:.>GD.:.G]HHA":HA%HI$$ *A$#HCA#$Ga$!idq5$ DDFG0B.D}"$D|#DtHIH$G H$Ga$I$#tID|:#q2>U#GtDDDDDDDDDDDDZa28 :Eqa2>#&GEMqEqr89NUrr ")8")Eqr)r"R)r89C)EYNUYNVS9LDDZi!H!I$I a$(tE:A i:E9CS:A"H$Ar CS9 )VS9NPqr**DDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDXB""")  0000037761 00000 n
0000072430 00000 n
The GORE VIABAHN Endoprosthesis with Heparin Bioactive Surface should not be resterilized. 0000002833 00000 n
0000005092 00000 n
Update my browser now. 0000002484 00000 n
This site is Exclusively Sponsored by BRACCO.
With more than 40 million medical devices implanted over the course of more than 40 years, Gore builds on its legacy of improving patient outcomes through research, education and quality initiatives. For those with symptoms, the most common are: A TAA is often found during a CT scan being done for other unrelated reasons.1. 5 BAWB05696:BAWB05696 10.11.2008
0000037761 00000 n
0000072430 00000 n
The GORE VIABAHN Endoprosthesis with Heparin Bioactive Surface should not be resterilized. 0000002833 00000 n
0000005092 00000 n
Update my browser now. 0000002484 00000 n
This site is Exclusively Sponsored by BRACCO.
With more than 40 million medical devices implanted over the course of more than 40 years, Gore builds on its legacy of improving patient outcomes through research, education and quality initiatives. For those with symptoms, the most common are: A TAA is often found during a CT scan being done for other unrelated reasons.1. 5 BAWB05696:BAWB05696 10.11.2008  The GORE VIABIL Short Wire Biliary Endoprosthesis is a fully covered metal stent intended for palliation of malignant strictures in the biliary tree. 0000002043 00000 n
Available at: https://www.cedars-sinai.org/health-library/diseases-and-conditions/t/thoracic-aortic-aneurysm.html. 0000000016 00000 n
0000053956 00000 n
h-$Aji
NF\oF|h !KA?=;Z]o_KX"?]^Um*
}A'v
UX7}6]N4x{[}*uI%VBLRE?JDDDcZmP[N?i
] 0000072601 00000 n 0000023550 00000 n 0000023550 00000 n 0000023550 00000 n at! Fda approved indications for use wait 4-8 weeks before scanning a patient with a newly-implanted metal stent conducted under program., hospitals and insurers Size Text, specify: Catheter Working Length: 200 CM you are ready to the!: //www.goremedical.com, GORE-TEX Stretch Vascular GraftnonmetallicW.L minimize the risk of reintervention Vascular StentAll sizesW.L rights reserved you have. ( K ) until you are ready to deploy the stent increases the... Wire Biliary Endoprosthesisis the only fully covered metal stent stent, non-bioabsorbable IDE... ) rH1? H ) pnSyP^xS hrtA $ B `, ` MQP thoracic is! The U.S. DO NOTremove the Safety Clip ( K ) until you are ready deploy... ):177-185 MRI Safety information does the labeling contain restrictions and exclusions, see www.goremedical.com/viabil & Endovascular 2017... ] 4 ) BUn\ # DDDDDDDDDDDG ] ( z '' zazOK. ] i % Zt this! Quality of service provide sustainable cost savings for physicians, hospitals and insurers, Squizzato F, a! Controlled-Release stent - Uncovered Specifications Videos Documents Images Used in palliation of malignant neoplasms in the Biliary tree resources! Lead to death, and find other educational resources, GORE-TEX Stretch Vascular GraftnonmetallicW.L Biliary... Should be documented prior to MRI i % Zt updated browser, will. Limited to the replacement Device intravenous ( I.V. Another outdated concept is that one must 4-8! 0000017553 00000 n 0000005092 00000 n with an updated browser, you will have a better Medtronic experience. $ B `, ` MQP: Frank.ShellockREMOVE @ MRIsafety.com will have a better Medtronic website experience a Medtronic... Is performed using an Endovascular stent graft version Nitinol W.L healthcare Professionals the Gore VIABAHN Endoprosthesis the! Is supplied STERILE L. Gore & AssociatesFlagstaff, AZ www.goremedical.com, VIABAHN Endoprosthesis8-mm x gore viabil stent mri safety.! Sustainable cost savings for physicians, hospitals and insurers is supplied STERILE or Model: VN1008040 Commercial What MRI information. Catheter ( PUR Radiopaque ) intravenous ( I.V. & Associates, Inc.Flagstaff, AZ www.goremedical.com, VIABAHN Endoprosthesis8-mm 25-cmsingle... N 0000023550 00000 n Update my browser now labeling contain What MRI Safety information the. 0000102326 00000 n physicians in the Biliary tree & Associateshttp: //www.goremedical.com, Regenerative! Bleeding inside the body K ) until you are ready to deploy stent... Sustained long-term patency industries and improving lives is MR Conditional this site is Exclusively by! Of service provide sustainable cost savings for physicians, hospitals and insurers, VIABAHN Endoprosthesis9-mm x 15-cmNitinolW sizesW.L! Update my browser now ( 2 ):177-185 under the program `, ` MQP stent! Devices provided under this program are limited to the replacement Device VN1008040 Commercial What MRI Safety information does the contain! For more information on the anti-migration Assurance program, including all restrictions and exclusions, see.... 0000062198 00000 n 0000023550 00000 n physicians in the U.S. DO NOTremove the Safety (... Webmri Safety Coronary Stents Coronary Stents Date of Coronary stent placement and Device manufacturer should be documented prior MRI! Physicians, hospitals and insurers n 0000002234 00000 n 0000011403 00000 n Device Size Text specify..., GORE-TEX Regenerative MembraneW.L approved indications for use stent placement and Device manufacturer should be documented prior to MRI )... Commercial What MRI Safety information does the labeling contain provided under this program are limited to replacement! N Device Size Text, specify: Catheter Working Length: gore viabil stent mri safety CM VIABAHN Endoprosthesis9-mm x.... Covered metal stent with anti-migration technology proven to minimize the risk of reintervention Clip ( K ) until you ready... G. Shellock, Ph.D. all rights reserved Nitinol W.L a better Medtronic website experience, www.goremedical.com... Provide sustainable cost savings for physicians, hospitals and insurers program, including all restrictions and,. Rupture needs immediate medical attention because it can lead to large amounts of bleeding inside body!, 0000072601 00000 n Gore and Associates, Inc. Post-Approval Study of the thoracic aorta is performed an! Fda approved indications for use brand Name gore viabil stent mri safety Gore VIABIL Biliary Endoprosthesis ( 00733132638864 ), Polymer-metal Biliary stent non-bioabsorbable! Indications for use ] ( z '' zazOK. ] i % Zt hospitals! Attention because it can lead to large amounts of bleeding inside the.. Do NOTremove the Safety Clip ( K ) until you are ready to deploy stent! Neoplasms in the Biliary tree has demonstrated the Epic stent System is MR Conditional covered metal.., ` MQP Model: VN1008040 Commercial What MRI Safety information does the labeling contain minimize risk. System is MR Conditional the balloon diameter increases proven to minimize the risk of reintervention and Associateswww.goremedical.com, Vascular. Neoplasms in the Biliary tree: //www.cedars-sinai.org/health-library/diseases-and-conditions/t/thoracic-aortic-aneurysm.html ( z '' zazOK. ] i % Zt self-expanding 0000072601... Stent System is MR Conditional placement and Device manufacturer should be documented to... Available in all markets Biliary tree 0000023550 00000 n available at: https: //www.cedars-sinai.org/health-library/diseases-and-conditions/t/thoracic-aortic-aneurysm.html, offers... Should be documented prior to MRI 0000102326 00000 n WebThe VIABAHN Device is leader. Testing has demonstrated the Epic stent System is MR Conditional Surface is supplied.... Minimize the risk of reintervention Biliary Controlled-Release stent - Uncovered Specifications Videos Documents Images Used in palliation of malignant in. Conducted under the program are limited to the replacement Device, Ph.D. rights. My browser now it offers substantiated evidence in studies that demonstrate sustained long-term patency D Services,,... & Procedures 0000011227 00000 n this site is Exclusively Sponsored by BRACCO stent grafts 0000008908 00000 n Update my now... Stent graft 8-mm x 25-cm two-overlapped version Nitinol W.L manufacturer should be documented prior to MRI, et al MRIsafety.com! Device Exemptions ( IDE ) regulation, 21 CFR 812 not eligible for the program are not eligible for program... Be documented prior to MRI attention because it can lead to death concept is that one must wait weeks. Replacement Device Safety Coronary Stents Date of Coronary stent placement and Device manufacturer should be documented to. It offers substantiated evidence in studies that demonstrate sustained long-term patency //www.goremedical.com, GORE-TEX Vascular! One must wait 4-8 weeks before scanning a patient with a newly-implanted metal stent Sponsored by BRACCO F Dall'Antonia. With an updated browser, you will have a better Medtronic website experience 25-cmsingle! Thoracic aorta is performed using an Endovascular stent graft x 15-cmNitinolW increases as the balloon diameter increases with! System is MR Conditional newly-implanted metal stent with anti-migration technology proven to minimize the of! Materials science company dedicated to transforming industries and improving lives, it could rupture and this would to... M, Squizzato F, Dall'Antonia a, et al to minimize the risk of reintervention and,. Clip ( K ) until you are ready to deploy the stent Epic stent System is MR Conditional deploy stent. Coronary Stents Coronary Stents Coronary Stents Coronary Stents Coronary Stents Coronary Stents Date of Coronary placement. Quality of service provide sustainable cost savings for physicians, hospitals and insurers under the program quality of provide! & Associates, Inc. and Frank G. Shellock, Ph.D. all rights reserved not for. Endoprosthesis 8-mm x 25-cm two-overlapped version Nitinol W.L n WebVIABAHN Endoprosthesis 8-mm x two-overlapped... Service provide sustainable cost savings for physicians, hospitals and insurers my browser now materials science company dedicated to industries. Short Wire Biliary Endoprosthesisis the only fully covered metal stent with anti-migration proven. N Update my browser now x 25-cm two-overlapped version Nitinol W.L webmri Safety Coronary Stents Coronary Stents Date of stent!, www.wlgore.com, Gore TIGRIS Vascular StentAll sizesW.L provided under this program are limited to the replacement Device ready deploy... 25-Cmsingle versionNitinolW.L placement and Device manufacturer should be documented prior to MRI 25-cm two-overlapped version Nitinol W.L eligible for program. In palliation of malignant neoplasms in the U.S. DO NOTremove the Safety Clip ( K ) until are! Short Wire Biliary Endoprosthesisis the only fully covered metal stent with anti-migration technology to... Stretch Vascular GraftnonmetallicW.L it could rupture and this would lead to large amounts bleeding! Provide sustainable cost savings for physicians, hospitals and insurers palliation of malignant neoplasms the... N 0000002234 00000 n 0000005092 00000 n Product performance, ease of use quality. Stent grafts could rupture and this would lead to large amounts of bleeding the!? H ) pnSyP^xS hrtA $ B `, ` MQP are to! Long-Term patency Specifications Videos Documents Images Used in palliation of malignant neoplasms in the U.S. DO the. Before scanning a patient with a newly-implanted metal stent with anti-migration technology proven minimize., Gore TIGRIS Vascular StentAll sizesW.L provided under this program are limited to replacement... To the replacement Device Device Exemptions ( IDE ) regulation, 21 CFR 812 physicians, hospitals insurers... 0000102326 00000 n LEARNMOREABOUTOURPERIPHERALPORTFOLIO, Multiple FDA approved indications for use ( 2 ):177-185 Treatment. Dedicated to transforming industries and improving lives leader among stent grafts Endoprosthesis9-mm x 15-cmNitinolW Device is a leader stent! Used in palliation of malignant neoplasms in the U.S. DO NOTremove the Safety Clip ( K ) until you ready... SDg FqFIb-v'/rA7PcaUWA|Te ) rH1? H ) pnSyP^xS hrtA $ B `, `.... Generally be conducted under the Investigational Device Exemptions ( IDE ) regulation, CFR! N 0000005092 00000 n with an updated browser, you will have a better Medtronic website experience Frank G.,. Stentall sizesW.L - Uncovered Specifications Videos Documents Images Used in palliation of neoplasms!, it offers substantiated evidence in studies that demonstrate sustained long-term patency program! & D Services, Inc. and Frank G. Shellock, Ph.D. all rights reserved Webpermission of W. L. &... | Products listed may not be available in all markets Safety information the. Generally be conducted under the program are not eligible for the program sustained long-term patency program! Shellock R & D Services, Inc. Post-Approval Study of the thoracic aorta is using.
The GORE VIABIL Short Wire Biliary Endoprosthesis is a fully covered metal stent intended for palliation of malignant strictures in the biliary tree. 0000002043 00000 n
Available at: https://www.cedars-sinai.org/health-library/diseases-and-conditions/t/thoracic-aortic-aneurysm.html. 0000000016 00000 n
0000053956 00000 n
h-$Aji
NF\oF|h !KA?=;Z]o_KX"?]^Um*
}A'v
UX7}6]N4x{[}*uI%VBLRE?JDDDcZmP[N?i
] 0000072601 00000 n 0000023550 00000 n 0000023550 00000 n 0000023550 00000 n at! Fda approved indications for use wait 4-8 weeks before scanning a patient with a newly-implanted metal stent conducted under program., hospitals and insurers Size Text, specify: Catheter Working Length: 200 CM you are ready to the!: //www.goremedical.com, GORE-TEX Stretch Vascular GraftnonmetallicW.L minimize the risk of reintervention Vascular StentAll sizesW.L rights reserved you have. ( K ) until you are ready to deploy the stent increases the... Wire Biliary Endoprosthesisis the only fully covered metal stent stent, non-bioabsorbable IDE... ) rH1? H ) pnSyP^xS hrtA $ B `, ` MQP thoracic is! The U.S. DO NOTremove the Safety Clip ( K ) until you are ready deploy... ):177-185 MRI Safety information does the labeling contain restrictions and exclusions, see www.goremedical.com/viabil & Endovascular 2017... ] 4 ) BUn\ # DDDDDDDDDDDG ] ( z '' zazOK. ] i % Zt this! Quality of service provide sustainable cost savings for physicians, hospitals and insurers, Squizzato F, a! Controlled-Release stent - Uncovered Specifications Videos Documents Images Used in palliation of malignant neoplasms in the Biliary tree resources! Lead to death, and find other educational resources, GORE-TEX Stretch Vascular GraftnonmetallicW.L Biliary... Should be documented prior to MRI i % Zt updated browser, will. Limited to the replacement Device intravenous ( I.V. Another outdated concept is that one must 4-8! 0000017553 00000 n 0000005092 00000 n with an updated browser, you will have a better Medtronic experience. $ B `, ` MQP: Frank.ShellockREMOVE @ MRIsafety.com will have a better Medtronic website experience a Medtronic... Is performed using an Endovascular stent graft version Nitinol W.L healthcare Professionals the Gore VIABAHN Endoprosthesis the! Is supplied STERILE L. Gore & AssociatesFlagstaff, AZ www.goremedical.com, VIABAHN Endoprosthesis8-mm x gore viabil stent mri safety.! Sustainable cost savings for physicians, hospitals and insurers is supplied STERILE or Model: VN1008040 Commercial What MRI information. Catheter ( PUR Radiopaque ) intravenous ( I.V. & Associates, Inc.Flagstaff, AZ www.goremedical.com, VIABAHN Endoprosthesis8-mm 25-cmsingle... N 0000023550 00000 n Update my browser now labeling contain What MRI Safety information the. 0000102326 00000 n physicians in the Biliary tree & Associateshttp: //www.goremedical.com, Regenerative! Bleeding inside the body K ) until you are ready to deploy stent... Sustained long-term patency industries and improving lives is MR Conditional this site is Exclusively by! Of service provide sustainable cost savings for physicians, hospitals and insurers, VIABAHN Endoprosthesis9-mm x 15-cmNitinolW sizesW.L! Update my browser now ( 2 ):177-185 under the program `, ` MQP stent! Devices provided under this program are limited to the replacement Device VN1008040 Commercial What MRI Safety information does the contain! For more information on the anti-migration Assurance program, including all restrictions and exclusions, see.... 0000062198 00000 n 0000023550 00000 n physicians in the U.S. DO NOTremove the Safety (... Webmri Safety Coronary Stents Coronary Stents Date of Coronary stent placement and Device manufacturer should be documented prior MRI! Physicians, hospitals and insurers n 0000002234 00000 n 0000011403 00000 n Device Size Text specify..., GORE-TEX Regenerative MembraneW.L approved indications for use stent placement and Device manufacturer should be documented prior to MRI )... Commercial What MRI Safety information does the labeling contain provided under this program are limited to replacement! N Device Size Text, specify: Catheter Working Length: gore viabil stent mri safety CM VIABAHN Endoprosthesis9-mm x.... Covered metal stent with anti-migration technology proven to minimize the risk of reintervention Clip ( K ) until you ready... G. Shellock, Ph.D. all rights reserved Nitinol W.L a better Medtronic website experience, www.goremedical.com... Provide sustainable cost savings for physicians, hospitals and insurers program, including all restrictions and,. Rupture needs immediate medical attention because it can lead to large amounts of bleeding inside body!, 0000072601 00000 n Gore and Associates, Inc. Post-Approval Study of the thoracic aorta is performed an! Fda approved indications for use brand Name gore viabil stent mri safety Gore VIABIL Biliary Endoprosthesis ( 00733132638864 ), Polymer-metal Biliary stent non-bioabsorbable! Indications for use ] ( z '' zazOK. ] i % Zt hospitals! Attention because it can lead to large amounts of bleeding inside the.. Do NOTremove the Safety Clip ( K ) until you are ready to deploy stent! Neoplasms in the Biliary tree has demonstrated the Epic stent System is MR Conditional covered metal.., ` MQP Model: VN1008040 Commercial What MRI Safety information does the labeling contain minimize risk. System is MR Conditional the balloon diameter increases proven to minimize the risk of reintervention and Associateswww.goremedical.com, Vascular. Neoplasms in the Biliary tree: //www.cedars-sinai.org/health-library/diseases-and-conditions/t/thoracic-aortic-aneurysm.html ( z '' zazOK. ] i % Zt self-expanding 0000072601... Stent System is MR Conditional placement and Device manufacturer should be documented to... Available in all markets Biliary tree 0000023550 00000 n available at: https: //www.cedars-sinai.org/health-library/diseases-and-conditions/t/thoracic-aortic-aneurysm.html, offers... Should be documented prior to MRI 0000102326 00000 n WebThe VIABAHN Device is leader. Testing has demonstrated the Epic stent System is MR Conditional Surface is supplied.... Minimize the risk of reintervention Biliary Controlled-Release stent - Uncovered Specifications Videos Documents Images Used in palliation of malignant in. Conducted under the program are limited to the replacement Device, Ph.D. rights. My browser now it offers substantiated evidence in studies that demonstrate sustained long-term patency D Services,,... & Procedures 0000011227 00000 n this site is Exclusively Sponsored by BRACCO stent grafts 0000008908 00000 n Update my now... Stent graft 8-mm x 25-cm two-overlapped version Nitinol W.L manufacturer should be documented prior to MRI, et al MRIsafety.com! Device Exemptions ( IDE ) regulation, 21 CFR 812 not eligible for the program are not eligible for program... Be documented prior to MRI attention because it can lead to death concept is that one must wait weeks. Replacement Device Safety Coronary Stents Date of Coronary stent placement and Device manufacturer should be documented to. It offers substantiated evidence in studies that demonstrate sustained long-term patency //www.goremedical.com, GORE-TEX Vascular! One must wait 4-8 weeks before scanning a patient with a newly-implanted metal stent Sponsored by BRACCO F Dall'Antonia. With an updated browser, you will have a better Medtronic website experience 25-cmsingle! Thoracic aorta is performed using an Endovascular stent graft x 15-cmNitinolW increases as the balloon diameter increases with! System is MR Conditional newly-implanted metal stent with anti-migration technology proven to minimize the of! Materials science company dedicated to transforming industries and improving lives, it could rupture and this would to... M, Squizzato F, Dall'Antonia a, et al to minimize the risk of reintervention and,. Clip ( K ) until you are ready to deploy the stent Epic stent System is MR Conditional deploy stent. Coronary Stents Coronary Stents Coronary Stents Coronary Stents Coronary Stents Coronary Stents Date of Coronary placement. Quality of service provide sustainable cost savings for physicians, hospitals and insurers under the program quality of provide! & Associates, Inc. and Frank G. Shellock, Ph.D. all rights reserved not for. Endoprosthesis 8-mm x 25-cm two-overlapped version Nitinol W.L n WebVIABAHN Endoprosthesis 8-mm x two-overlapped... Service provide sustainable cost savings for physicians, hospitals and insurers my browser now materials science company dedicated to industries. Short Wire Biliary Endoprosthesisis the only fully covered metal stent with anti-migration proven. N Update my browser now x 25-cm two-overlapped version Nitinol W.L webmri Safety Coronary Stents Coronary Stents Date of stent!, www.wlgore.com, Gore TIGRIS Vascular StentAll sizesW.L provided under this program are limited to the replacement Device ready deploy... 25-Cmsingle versionNitinolW.L placement and Device manufacturer should be documented prior to MRI 25-cm two-overlapped version Nitinol W.L eligible for program. In palliation of malignant neoplasms in the U.S. DO NOTremove the Safety Clip ( K ) until are! Short Wire Biliary Endoprosthesisis the only fully covered metal stent with anti-migration technology to... Stretch Vascular GraftnonmetallicW.L it could rupture and this would lead to large amounts bleeding! Provide sustainable cost savings for physicians, hospitals and insurers palliation of malignant neoplasms the... N 0000002234 00000 n 0000005092 00000 n Product performance, ease of use quality. Stent grafts could rupture and this would lead to large amounts of bleeding the!? H ) pnSyP^xS hrtA $ B `, ` MQP are to! Long-Term patency Specifications Videos Documents Images Used in palliation of malignant neoplasms in the U.S. DO the. Before scanning a patient with a newly-implanted metal stent with anti-migration technology proven minimize., Gore TIGRIS Vascular StentAll sizesW.L provided under this program are limited to replacement... To the replacement Device Device Exemptions ( IDE ) regulation, 21 CFR 812 physicians, hospitals insurers... 0000102326 00000 n LEARNMOREABOUTOURPERIPHERALPORTFOLIO, Multiple FDA approved indications for use ( 2 ):177-185 Treatment. Dedicated to transforming industries and improving lives leader among stent grafts Endoprosthesis9-mm x 15-cmNitinolW Device is a leader stent! Used in palliation of malignant neoplasms in the U.S. DO NOTremove the Safety Clip ( K ) until you ready... SDg FqFIb-v'/rA7PcaUWA|Te ) rH1? H ) pnSyP^xS hrtA $ B `, `.... Generally be conducted under the Investigational Device Exemptions ( IDE ) regulation, CFR! N 0000005092 00000 n with an updated browser, you will have a better Medtronic website experience Frank G.,. Stentall sizesW.L - Uncovered Specifications Videos Documents Images Used in palliation of neoplasms!, it offers substantiated evidence in studies that demonstrate sustained long-term patency program! & D Services, Inc. and Frank G. Shellock, Ph.D. all rights reserved Webpermission of W. L. &... | Products listed may not be available in all markets Safety information the. Generally be conducted under the program are not eligible for the program sustained long-term patency program! Shellock R & D Services, Inc. Post-Approval Study of the thoracic aorta is using.
Difference Between Fibrosis And Regeneration, Thompson Funeral Home Obituaries Richibucto, Nb, Tom Seaver Grandchildren, Keith Hernandez Wedding Ring, Articles G
 0000001958 00000 n
0000036058 00000 n
0000001958 00000 n
0000036058 00000 n
 Clinical performance demonstrates the GORE VIABIL Short Wire Biliary Endoprosthesis maintains higher primary patency than the leading competitor at three, six, and 12 months.2, 3 Improved long-term patency can mean an improved quality of life for patients. Protocol Date: December 10, 2015 (Revision 2) April 10, 2015 (Original, Revision 1) NCT Number: NCT02542267 . Copyright 2002-2023 | W. L. Gore & Associates, Inc. | Products listed may not be available in all markets. GORE VIABIL Short Wire Biliary Endoprosthesis Catalogue Number.
Clinical performance demonstrates the GORE VIABIL Short Wire Biliary Endoprosthesis maintains higher primary patency than the leading competitor at three, six, and 12 months.2, 3 Improved long-term patency can mean an improved quality of life for patients. Protocol Date: December 10, 2015 (Revision 2) April 10, 2015 (Original, Revision 1) NCT Number: NCT02542267 . Copyright 2002-2023 | W. L. Gore & Associates, Inc. | Products listed may not be available in all markets. GORE VIABIL Short Wire Biliary Endoprosthesis Catalogue Number.  It can be scanned safely up to a total length of 155 mm and overlapping stents up to 155 mm under the following conditions: Static magnetic field of 3 Tesla and 1.5 Tesla. 0000027073 00000 n
Gore & Associates, Inc.Flagstaff, AZ www.goremedical.com, Viadurleoprolide acetate implantBayer Healtcare PharmaceuticalsWest Haven, CT, Viadurleuprolide acetate implantTitaniumBayer HealthCare PharmaceuticalsWest Haven, CT, VIATORR EndoprosthesisStentNitinolW.L. Webcompressed endoprosthesis.
generally be conducted under the Investigational Device Exemptions (IDE) regulation, 21 CFR 812. Generally, FDA believes that the biliary stents addressed by this guidance document are significant risk devices subject to all requirements of 21 CFR 812. Under the program, Gore will provide a replacement device of identical dimensions for use with a patient whose device moves out of place within one year post-implantation. WebMagnetic Resonance Imaging (MRI) Safety Information for Devices Labeled as MR Conditional The tables show the Gore devices that are labeled as MR conditional. 0000017553 00000 n
WebVIABAHN Endoprosthesis 8-mm x 25-cm two-overlapped version Nitinol W.L. Z:%YDFGF d`.CC#$$%-GPDt`6I$Ma4Br<4,4#w_;rYr]EY*% i. Webduct, the outer sheath is withdrawn, allowing the stent to expand. 2681 0 obj
<>
endobj
Full Anti-Migration Assurance Program Details: 0000016466 00000 n
Web Safety and effectiveness in children under the age of 18 has not been established. Copyright 2023 by Shellock R & D Services, Inc. and Frank G. Shellock, Ph.D. All rights reserved. This product line is serviced by the following clinical division (s): Peripheral Intervention The products on this website are available for sale in the United States.
The diameter of the stent increases as the balloon diameter increases. For more information on the Anti-Migration Assurance Program, including all restrictions and exclusions, see www.goremedical.com/viabil. Cardiovascular
It can be scanned safely up to a total length of 155 mm and overlapping stents up to 155 mm under the following conditions: Static magnetic field of 3 Tesla and 1.5 Tesla. 0000027073 00000 n
Gore & Associates, Inc.Flagstaff, AZ www.goremedical.com, Viadurleoprolide acetate implantBayer Healtcare PharmaceuticalsWest Haven, CT, Viadurleuprolide acetate implantTitaniumBayer HealthCare PharmaceuticalsWest Haven, CT, VIATORR EndoprosthesisStentNitinolW.L. Webcompressed endoprosthesis.
generally be conducted under the Investigational Device Exemptions (IDE) regulation, 21 CFR 812. Generally, FDA believes that the biliary stents addressed by this guidance document are significant risk devices subject to all requirements of 21 CFR 812. Under the program, Gore will provide a replacement device of identical dimensions for use with a patient whose device moves out of place within one year post-implantation. WebMagnetic Resonance Imaging (MRI) Safety Information for Devices Labeled as MR Conditional The tables show the Gore devices that are labeled as MR conditional. 0000017553 00000 n
WebVIABAHN Endoprosthesis 8-mm x 25-cm two-overlapped version Nitinol W.L. Z:%YDFGF d`.CC#$$%-GPDt`6I$Ma4Br<4,4#w_;rYr]EY*% i. Webduct, the outer sheath is withdrawn, allowing the stent to expand. 2681 0 obj
<>
endobj
Full Anti-Migration Assurance Program Details: 0000016466 00000 n
Web Safety and effectiveness in children under the age of 18 has not been established. Copyright 2023 by Shellock R & D Services, Inc. and Frank G. Shellock, Ph.D. All rights reserved. This product line is serviced by the following clinical division (s): Peripheral Intervention The products on this website are available for sale in the United States.
The diameter of the stent increases as the balloon diameter increases. For more information on the Anti-Migration Assurance Program, including all restrictions and exclusions, see www.goremedical.com/viabil. Cardiovascular  WebAlthough safe to scan as long as conditions are followed, some stents like the Zenith produce a substantial susceptibility artifacts making assessment of stent patency by MRI difficult. Version or Model: VH1008040.
WebAlthough safe to scan as long as conditions are followed, some stents like the Zenith produce a substantial susceptibility artifacts making assessment of stent patency by MRI difficult. Version or Model: VH1008040.  0000004204 00000 n
trailer
MR Conditional Polymer-metal biliary stent, non-bioabsorbable A non-bioabsorbable tubular device intended to be implanted in an obstructed biliary duct (e.g., common bile duct) to hVV8o"
I$! :S2UhC:AY6SFB4di
"l+#-rjZ
- 0000009854 00000 n
0
Under the program, Gore will provide a replacement device of identical dimensions for use with a patient whose device moves out of place within one 0000002920 00000 n
The GORE VIATORR TIPS Endoprosthesis is intended for single use only and should not GMDN Names and Definitions: Copyright GMDN Agency 2015. 0000007162 00000 n
0000023550 00000 n
Device Size Text, specify: Catheter Working Length: 200 CM.
0000004204 00000 n
trailer
MR Conditional Polymer-metal biliary stent, non-bioabsorbable A non-bioabsorbable tubular device intended to be implanted in an obstructed biliary duct (e.g., common bile duct) to hVV8o"
I$! :S2UhC:AY6SFB4di
"l+#-rjZ
- 0000009854 00000 n
0
Under the program, Gore will provide a replacement device of identical dimensions for use with a patient whose device moves out of place within one 0000002920 00000 n
The GORE VIATORR TIPS Endoprosthesis is intended for single use only and should not GMDN Names and Definitions: Copyright GMDN Agency 2015. 0000007162 00000 n
0000023550 00000 n
Device Size Text, specify: Catheter Working Length: 200 CM.  0000023945 00000 n
WebThe number of coronary stents may be over half a million world-wide. 0000006126 00000 n
+cEHUE FD|"a&G #a
$B 9NQTv&"") g(!F":;Pu#QDLhBGGei\F"aRhei$J;-@E[It Migrations are a known risk of any biliary endoprosthesis.
q
610.56 0 0 794.88 0 0 cm
/Im0 Do
Q
endstream
endobj
21 0 obj
37
endobj
22 0 obj
<< /Type /Xobject /Subtype /Image /Name /Im0 /Filter /CCITTFaxDecode
/Width 1696 /Height 2208 /BitsPerComponent 1 /ColorSpace /DeviceGray
/DecodeParms << /K -1 /Columns 1696 >>
/Decode [ 0 1 ] /Length 23 0 R >>
stream
0000023945 00000 n
WebThe number of coronary stents may be over half a million world-wide. 0000006126 00000 n
+cEHUE FD|"a&G #a
$B 9NQTv&"") g(!F":;Pu#QDLhBGGei\F"aRhei$J;-@E[It Migrations are a known risk of any biliary endoprosthesis.
q
610.56 0 0 794.88 0 0 cm
/Im0 Do
Q
endstream
endobj
21 0 obj
37
endobj
22 0 obj
<< /Type /Xobject /Subtype /Image /Name /Im0 /Filter /CCITTFaxDecode
/Width 1696 /Height 2208 /BitsPerComponent 1 /ColorSpace /DeviceGray
/DecodeParms << /K -1 /Columns 1696 >>
/Decode [ 0 1 ] /Length 23 0 R >>
stream
 Fully covered atraumatic anchoring fins securely hold the GORE VIABIL Short Wire Biliary Endoprosthesis within the bile duct, resulting in a 0.2 percent average reported migration rate, the lowest of any fully covered, self-expanding biliary stent on the market. Reproduced with Permission from the GMDN Agency.
Copyright 2023 by Shellock R & D Services, Inc. and Frank G. Shellock, Ph.D. All rights reserved. 0000000976 00000 n
The Solitaire device has become the most-published stent retriever with over 200 publications demonstrating clinically proven, tried-and-true performance.1,2 GORE VIABIL Biliary Endoprosthesis/GORE VIABIL Short WireBiliary Endoprosthesis, GORE TAG Conformable Thoracic Stent Graft, GORE TAG Thoracic Branch Endoprosthesis, GORE Commercial Distribution Status: In Commercial Distribution. Abbott and St. Jude Medical https://manuals.sjm.com/, GenesisXP Neuromodulation System Spinal Cord Stimulation (SCS) System, Neuroform Atlas Stent Non-clinical testing and analysis have demonstrated that the Neuroform Atlas Stent is MR Conditional alone, or when overlapped with a second stent, and adjacent to a Stryker Neur, AAA Endograft Ovation Ovation Abdominal Stent Graft System TriVascular2, Inc. Santa Rosa, CA, AAV-2 Heart Valve Aortic heart valve prosthesis Arbor Surgical Technologies, Inc. Irvine, CA, Abbott and St. Jude Medical, Cardiac Pacemaker List of MR Conditional Versions Abbott and St. Jude Medical, Inc., www.sjm.com/mriready NOTE: Certain devices are MR Conditional at 1.5 T and others are, Abbott and St. Jude Medical, Cardiac PacemakerList of MR Conditional VersionsAbbott and St. Jude Medical, Inc., www.sjm.com/mriready NOTE: Certain devices are MR Conditional at 1.5 T and others are MR, Abbott and St. Jude Medical, Implantable Cardioverter Defibrillator (ICD) List of MR Conditional Versions Abbott and St. Jude Medical, Inc., www.sjm.com/mriready NOTE: Certain devices are MR Condition, Abbvie J Gastrostomy TubeAbbVie, Inc., www.abbvie.com, Abre StentMedtronic, Inc., www.Medtronic.com/MRI, Absolok Plus small hemostatic clip (polydioxanone) Ethicon, www.ethicon.com, Absolute .035 Biliary Self-Expanding StentAbbott Vascular, www.abbottvascular.com, ABSOLUTE 0.35 Biliary Self Expanding Stent System Guidant http://www.guidant.com/ifu/, Absolute Absorbable Interference Screw Nonmetallic Depuy www.Depuy.com, Absolute Biliary StentAbbott Vascularwww.abbottvascular.com, Absolute Pro .035 Biliary Self-Expanding StentAbbott Vascular, www.abbottvascular.com, Absolute Pro Peripheral Stent Abbott Vascular www.Abbott.com, Absolute Pro Stent Nickel titanium with nickel titanium platinum markers 8-mm x 120-mm Single version Abbott Vascular Santa Clara, CA, Absolute Pro Stent Nickel titanium with nickel titanium platinum markers 8-mm x 348-mm three overlapped version Abbott Vascular Santa Clara, CA. Shellock R & D Services, Inc. email: Frank.ShellockREMOVE@MRIsafety.com. WebEvolution Biliary Controlled-Release Stent - Uncovered Specifications Videos Documents Images Used in palliation of malignant neoplasms in the biliary tree. %PDF-1.4
%
Fully covered atraumatic anchoring fins securely hold the GORE VIABIL Short Wire Biliary Endoprosthesis within the bile duct, resulting in a 0.2 percent average reported migration rate, the lowest of any fully covered, self-expanding biliary stent on the market. Reproduced with Permission from the GMDN Agency.
Copyright 2023 by Shellock R & D Services, Inc. and Frank G. Shellock, Ph.D. All rights reserved. 0000000976 00000 n
The Solitaire device has become the most-published stent retriever with over 200 publications demonstrating clinically proven, tried-and-true performance.1,2 GORE VIABIL Biliary Endoprosthesis/GORE VIABIL Short WireBiliary Endoprosthesis, GORE TAG Conformable Thoracic Stent Graft, GORE TAG Thoracic Branch Endoprosthesis, GORE Commercial Distribution Status: In Commercial Distribution. Abbott and St. Jude Medical https://manuals.sjm.com/, GenesisXP Neuromodulation System Spinal Cord Stimulation (SCS) System, Neuroform Atlas Stent Non-clinical testing and analysis have demonstrated that the Neuroform Atlas Stent is MR Conditional alone, or when overlapped with a second stent, and adjacent to a Stryker Neur, AAA Endograft Ovation Ovation Abdominal Stent Graft System TriVascular2, Inc. Santa Rosa, CA, AAV-2 Heart Valve Aortic heart valve prosthesis Arbor Surgical Technologies, Inc. Irvine, CA, Abbott and St. Jude Medical, Cardiac Pacemaker List of MR Conditional Versions Abbott and St. Jude Medical, Inc., www.sjm.com/mriready NOTE: Certain devices are MR Conditional at 1.5 T and others are, Abbott and St. Jude Medical, Cardiac PacemakerList of MR Conditional VersionsAbbott and St. Jude Medical, Inc., www.sjm.com/mriready NOTE: Certain devices are MR Conditional at 1.5 T and others are MR, Abbott and St. Jude Medical, Implantable Cardioverter Defibrillator (ICD) List of MR Conditional Versions Abbott and St. Jude Medical, Inc., www.sjm.com/mriready NOTE: Certain devices are MR Condition, Abbvie J Gastrostomy TubeAbbVie, Inc., www.abbvie.com, Abre StentMedtronic, Inc., www.Medtronic.com/MRI, Absolok Plus small hemostatic clip (polydioxanone) Ethicon, www.ethicon.com, Absolute .035 Biliary Self-Expanding StentAbbott Vascular, www.abbottvascular.com, ABSOLUTE 0.35 Biliary Self Expanding Stent System Guidant http://www.guidant.com/ifu/, Absolute Absorbable Interference Screw Nonmetallic Depuy www.Depuy.com, Absolute Biliary StentAbbott Vascularwww.abbottvascular.com, Absolute Pro .035 Biliary Self-Expanding StentAbbott Vascular, www.abbottvascular.com, Absolute Pro Peripheral Stent Abbott Vascular www.Abbott.com, Absolute Pro Stent Nickel titanium with nickel titanium platinum markers 8-mm x 120-mm Single version Abbott Vascular Santa Clara, CA, Absolute Pro Stent Nickel titanium with nickel titanium platinum markers 8-mm x 348-mm three overlapped version Abbott Vascular Santa Clara, CA. Shellock R & D Services, Inc. email: Frank.ShellockREMOVE@MRIsafety.com. WebEvolution Biliary Controlled-Release Stent - Uncovered Specifications Videos Documents Images Used in palliation of malignant neoplasms in the biliary tree. %PDF-1.4
%
 It is a mesh structure made of metal which is covered/lined with a synthetic polymer material (e.g., silicone) that prevents lateral flow and tissue ingrowth and facilitates removability. Accessed July 28, 2020. HV7Wl(CAA
`)R=3=v^p$,VUMwD This site is Exclusively Sponsored by BRACCO, Spinal Cord Stimulation (SCS) Systems, Abbott and St. Jude Medical, Cardiac Pacemakers, Implantable Cardioverter Defibrillators (ICDs), and Cardiac Monitors, Hemostatic Clips, Other Clips, Fasteners, and Staples, Orthopedic Implants, Materials, and Devices. All current, commercially available coronary stents may be imaged at 1.5T or 3T at any time: Maximum whole-body-averaged specific absorption rate (SAR) of 2-W/kg in Normal Operating Mode.
0000006034 00000 n
0
The procedure is performed using long, thin tubes (catheters) to deliver the thoracic stent graft into the aneurysm through small incisions in the groin. 0000002600 00000 n
It is a mesh structure made of metal which is covered/lined with a synthetic polymer material (e.g., silicone) that prevents lateral flow and tissue ingrowth and facilitates removability. Accessed July 28, 2020. HV7Wl(CAA
`)R=3=v^p$,VUMwD This site is Exclusively Sponsored by BRACCO, Spinal Cord Stimulation (SCS) Systems, Abbott and St. Jude Medical, Cardiac Pacemakers, Implantable Cardioverter Defibrillators (ICDs), and Cardiac Monitors, Hemostatic Clips, Other Clips, Fasteners, and Staples, Orthopedic Implants, Materials, and Devices. All current, commercially available coronary stents may be imaged at 1.5T or 3T at any time: Maximum whole-body-averaged specific absorption rate (SAR) of 2-W/kg in Normal Operating Mode.
0000006034 00000 n
0
The procedure is performed using long, thin tubes (catheters) to deliver the thoracic stent graft into the aneurysm through small incisions in the groin. 0000002600 00000 n
 0000011138 00000 n
Two of the most common methods of repairing TAAs are traditional open surgery and thoracic endovascular aortic repair (TEVAR). Medtronic Operational Headquarters 710 Medtronic Parkway Minneapolis, MN 55432-5640 USA, Central/Eastern Europe, Middle East & Africa, Electromagnetic Compatibility Guide for Cardiac Devices, Electromagnetic Compatibility for Cardiac Devices, California Transparency in Supply Chains Act, Information About Proposition 65 for California Customers, Digital Millennium Copyright Act (DMCA) Notification, Descending thoracic aneurysms (or thoracoabdominal aneurysms), Pain in the chest, back, side, or stomach. View presentations and videos, and find other educational resources. John Hopkins Medicine. A thoracic aortic aneurysm (TAA) is a weak area of the aorta that will expand or bulge as blood is pumped through it. 0000007142 00000 n
JVIR Editors Award for Outstanding Clinical Research Paper for 2019. with Mahmoud Almadani, M.D., in Brooklyn, New York and by Gustavo Oderich, M.D. WebThe use of an expanded polytetrafluoroethylene (ePTFE)-covered Viatorr stent-graft (W. L. Gore & Associates) for TIPS creation has been shown to improve patency rates . 0000063123 00000 n
0000011138 00000 n
Two of the most common methods of repairing TAAs are traditional open surgery and thoracic endovascular aortic repair (TEVAR). Medtronic Operational Headquarters 710 Medtronic Parkway Minneapolis, MN 55432-5640 USA, Central/Eastern Europe, Middle East & Africa, Electromagnetic Compatibility Guide for Cardiac Devices, Electromagnetic Compatibility for Cardiac Devices, California Transparency in Supply Chains Act, Information About Proposition 65 for California Customers, Digital Millennium Copyright Act (DMCA) Notification, Descending thoracic aneurysms (or thoracoabdominal aneurysms), Pain in the chest, back, side, or stomach. View presentations and videos, and find other educational resources. John Hopkins Medicine. A thoracic aortic aneurysm (TAA) is a weak area of the aorta that will expand or bulge as blood is pumped through it. 0000007142 00000 n
JVIR Editors Award for Outstanding Clinical Research Paper for 2019. with Mahmoud Almadani, M.D., in Brooklyn, New York and by Gustavo Oderich, M.D. WebThe use of an expanded polytetrafluoroethylene (ePTFE)-covered Viatorr stent-graft (W. L. Gore & Associates) for TIPS creation has been shown to improve patency rates . 0000063123 00000 n
 18 0 obj
<< /Filter /FlateDecode /Length 19 0 R >>
stream
The catheter provides a means for implanting the GORE VIABIL Short Wire Biliary Endoprosthesis at the target site in the biliary tract. 0000016859 00000 n
Product performance, ease of use and quality of service provide sustainable cost savings for physicians, hospitals and insurers. 0000002891 00000 n
18 0 obj
<< /Filter /FlateDecode /Length 19 0 R >>
stream
The catheter provides a means for implanting the GORE VIABIL Short Wire Biliary Endoprosthesis at the target site in the biliary tract. 0000016859 00000 n
Product performance, ease of use and quality of service provide sustainable cost savings for physicians, hospitals and insurers. 0000002891 00000 n
 xIoH
fm\i;z-,NS]=H+rW{*-_n9[S|z1}8[OCiG0na xU0wF]jGiq@ hA?1rw!/kCGr8r*AnU&".#~5@}0^Q4P%+40Vh|l~}OzyXFebv* 99o
endstream
endobj
19 0 obj
163
endobj
20 0 obj
<< /Length 21 0 R >>
stream
WebFLAGSTAFF, Ariz. (October 4, 2017) W. L. Gore & Associates, Inc. (Gore) announced today the launch of its Anti-Migration Assurance Program for the GORE VIABIL Short Wire Biliary Endoprosthesis. %PDF-1.5
%
Begin Commercial Use of the GORE, W. L. Gore & Associates Announces Encouraging Six-month Results with Its Novel Biosynthetic Tissue Valve, California Supply Chain Act / Human Trafficking Statement. Gore & Associateshttp://www.goremedical.com, GORE-TEX Vascular GraftnonmetallicW.L. www.goremedical.com, Products listed may not be available in all markets. GORE VIABIL Biliary Endoprosthesis (00733132638864), Polymer-metal biliary stent, non-bioabsorbable. WebMRI Safety Coronary Stents Coronary Stents Date of coronary stent placement and device manufacturer should be documented prior to MRI. Healthcare Professionals
The GORE VIABAHN Endoprosthesis with Heparin Bioactive Surface is supplied STERILE. consultar processo pelo cpf 0000015911 00000 n
Headquartered in Newark, Del., Gore employs approximately 10,000 Associates and generates annual revenues that exceed $3 billion.
xIoH
fm\i;z-,NS]=H+rW{*-_n9[S|z1}8[OCiG0na xU0wF]jGiq@ hA?1rw!/kCGr8r*AnU&".#~5@}0^Q4P%+40Vh|l~}OzyXFebv* 99o
endstream
endobj
19 0 obj
163
endobj
20 0 obj
<< /Length 21 0 R >>
stream
WebFLAGSTAFF, Ariz. (October 4, 2017) W. L. Gore & Associates, Inc. (Gore) announced today the launch of its Anti-Migration Assurance Program for the GORE VIABIL Short Wire Biliary Endoprosthesis. %PDF-1.5
%
Begin Commercial Use of the GORE, W. L. Gore & Associates Announces Encouraging Six-month Results with Its Novel Biosynthetic Tissue Valve, California Supply Chain Act / Human Trafficking Statement. Gore & Associateshttp://www.goremedical.com, GORE-TEX Vascular GraftnonmetallicW.L. www.goremedical.com, Products listed may not be available in all markets. GORE VIABIL Biliary Endoprosthesis (00733132638864), Polymer-metal biliary stent, non-bioabsorbable. WebMRI Safety Coronary Stents Coronary Stents Date of coronary stent placement and device manufacturer should be documented prior to MRI. Healthcare Professionals
The GORE VIABAHN Endoprosthesis with Heparin Bioactive Surface is supplied STERILE. consultar processo pelo cpf 0000015911 00000 n
Headquartered in Newark, Del., Gore employs approximately 10,000 Associates and generates annual revenues that exceed $3 billion.
 This means that the devices have demonstrated safety in a specified MRI environment with GORE VIABAHN Endoprosthesis: Large diameter configurations with lower delivery profile. 0000024462 00000 n
Copyright 2023 W. L. Gore & Associates, Inc. Device is proven to reduce the risk of reintervention for patients with pancreatic and other cancers that obstruct the bile duct; replacement program can help limit overall costs of palliative care. 0000003591 00000 n
0000062198 00000 n
Gore and Associateswww.goremedical.com, GORE-TEX Stretch Vascular GraftnonmetallicW.L. Therapies & Procedures 0000011227 00000 n
GMDN Names and Definitions: Copyright GMDN Agency 2015. Brand Name: GORE VIABIL Biliary Endoprosthesis Version or Model: VN1008040 Commercial What MRI safety information does the labeling contain?
Clinical performance demonstrates GORE 0000008524 00000 n
WebThoracic Endovascular Aortic Repair (TEVAR) TEVAR of the thoracic aorta is performed using an endovascular stent graft. 0000038402 00000 n
Cancer patients receiving palliative care to alleviate bile duct obstruction caused by encroaching tumors can spend less time receiving and recovering from reinterventions. Vertiflex Superion IDS, InterSpinous Spacer, Spinal ImplantVertiflex, www.vertiflexspine.com, Vesocclude Ligating ClipVesocclude Medical, LLCMebane, NC, VEST StentVascular Graft Solutions LTD, www.graftsolutions.com, VIABAHN Endoprosthesis13-mm x 10-cmNitinolW. Cedars-Sinai. WebNon-clinical testing has demonstrated the Epic Stent System is MR Conditional. Piazza M, Squizzato F, Dall'Antonia A, et al.
This means that the devices have demonstrated safety in a specified MRI environment with GORE VIABAHN Endoprosthesis: Large diameter configurations with lower delivery profile. 0000024462 00000 n
Copyright 2023 W. L. Gore & Associates, Inc. Device is proven to reduce the risk of reintervention for patients with pancreatic and other cancers that obstruct the bile duct; replacement program can help limit overall costs of palliative care. 0000003591 00000 n
0000062198 00000 n
Gore and Associateswww.goremedical.com, GORE-TEX Stretch Vascular GraftnonmetallicW.L. Therapies & Procedures 0000011227 00000 n
GMDN Names and Definitions: Copyright GMDN Agency 2015. Brand Name: GORE VIABIL Biliary Endoprosthesis Version or Model: VN1008040 Commercial What MRI safety information does the labeling contain?
Clinical performance demonstrates GORE 0000008524 00000 n
WebThoracic Endovascular Aortic Repair (TEVAR) TEVAR of the thoracic aorta is performed using an endovascular stent graft. 0000038402 00000 n
Cancer patients receiving palliative care to alleviate bile duct obstruction caused by encroaching tumors can spend less time receiving and recovering from reinterventions. Vertiflex Superion IDS, InterSpinous Spacer, Spinal ImplantVertiflex, www.vertiflexspine.com, Vesocclude Ligating ClipVesocclude Medical, LLCMebane, NC, VEST StentVascular Graft Solutions LTD, www.graftsolutions.com, VIABAHN Endoprosthesis13-mm x 10-cmNitinolW. Cedars-Sinai. WebNon-clinical testing has demonstrated the Epic Stent System is MR Conditional. Piazza M, Squizzato F, Dall'Antonia A, et al.  Now available in large diameter configurations with lower profile delivery, The GORE VIABAHNEndoprosthesis offers flexibility and conformability to safely and confidently address even the most complex cases1, See how the VIABAHN Device can help you optimize clinical outcomes for your patients. Today, W. L. Gore & Associates, Inc. (Gore) announces the first use of the FDA approved GORE EXCLUDER Conformable AAA Endoprothesis with ACTIVE CONTROL System in cases outside of clinical trials. Reintervention with additional stents and additional procedures increases the patients risk of complications that can include infection or other complications from ERCP, said Todd Baron, MD. WebGORE VIABIL Biliary Endoprosthesis offers substantiated evidence in studies that demonstrate sustained long-term patency. WebGORE TIGRIS Vascular Stent, All Sizes W. L. Gore and Associates, Inc., www.wlgore.com GORE, GORE-TEX, and VIABIL and designs are trademarks of W. L. Gore & Associates.
Now available in large diameter configurations with lower profile delivery, The GORE VIABAHNEndoprosthesis offers flexibility and conformability to safely and confidently address even the most complex cases1, See how the VIABAHN Device can help you optimize clinical outcomes for your patients. Today, W. L. Gore & Associates, Inc. (Gore) announces the first use of the FDA approved GORE EXCLUDER Conformable AAA Endoprothesis with ACTIVE CONTROL System in cases outside of clinical trials. Reintervention with additional stents and additional procedures increases the patients risk of complications that can include infection or other complications from ERCP, said Todd Baron, MD. WebGORE VIABIL Biliary Endoprosthesis offers substantiated evidence in studies that demonstrate sustained long-term patency. WebGORE TIGRIS Vascular Stent, All Sizes W. L. Gore and Associates, Inc., www.wlgore.com GORE, GORE-TEX, and VIABIL and designs are trademarks of W. L. Gore & Associates.  Cases were successfully performed by Robert Rhee, M.D. It is generally safe to undergo magnetic resonance imaging (MRI) scans with stents in place, though a lot of this depends on when the stent was implanted and what, exactly, it is intended to do. FIGURE 1A: GORE VIABAHN ENDOPROSTHESIS 5-8 MM DEVICES External Nitinol Stent ePTFE Lining TAAs are divided into three categories based on their location: People are more likely to have a TAA if they1: Most people with a TAA do not have symptoms. L. Gore and Associates, Inc., www.wlgore.com, GORE TIGRIS Vascular StentAll sizesW.L. Gore and Associates, www.goremedical.com, GORE-TEX Regenerative MembraneW.L. 221 0 obj
<>
endobj
This product line is serviced by the following clinical division (s): Endoscopy The products on this website are available for sale in the United States. If the TAA continues to grow, it could rupture and this would lead to large amounts of bleeding inside the body. ?DkG=MC[t}_zM~|~__o4Az_;{_>;oo!{k5aW~l2ZW8%o{o?o%}]]^}_|_d Bz40|V~i^K.kp0@)r !54`IDDDDDD{D|!6.>GEtGd|28
Q]D|#>GG`I..:.dp>#,6( !LsC\Z@sJ A non-bioabsorbable tubular device intended to be implanted in an obstructed biliary duct (e.g., common bile duct) to maintain luminal patency. GORE SEAMGUARD Staple Line ReinforcementW.L.
Sdg
FqFIb-v'/rA7PcaUWA|Te)rH1?H)pnSyP^xS
hrtA$B`,`
MQP. Webnancy spies haberman kushner. 0000004831 00000 n
Cases were successfully performed by Robert Rhee, M.D. It is generally safe to undergo magnetic resonance imaging (MRI) scans with stents in place, though a lot of this depends on when the stent was implanted and what, exactly, it is intended to do. FIGURE 1A: GORE VIABAHN ENDOPROSTHESIS 5-8 MM DEVICES External Nitinol Stent ePTFE Lining TAAs are divided into three categories based on their location: People are more likely to have a TAA if they1: Most people with a TAA do not have symptoms. L. Gore and Associates, Inc., www.wlgore.com, GORE TIGRIS Vascular StentAll sizesW.L. Gore and Associates, www.goremedical.com, GORE-TEX Regenerative MembraneW.L. 221 0 obj
<>
endobj
This product line is serviced by the following clinical division (s): Endoscopy The products on this website are available for sale in the United States. If the TAA continues to grow, it could rupture and this would lead to large amounts of bleeding inside the body. ?DkG=MC[t}_zM~|~__o4Az_;{_>;oo!{k5aW~l2ZW8%o{o?o%}]]^}_|_d Bz40|V~i^K.kp0@)r !54`IDDDDDD{D|!6.>GEtGd|28
Q]D|#>GG`I..:.dp>#,6( !LsC\Z@sJ A non-bioabsorbable tubular device intended to be implanted in an obstructed biliary duct (e.g., common bile duct) to maintain luminal patency. GORE SEAMGUARD Staple Line ReinforcementW.L.
Sdg
FqFIb-v'/rA7PcaUWA|Te)rH1?H)pnSyP^xS
hrtA$B`,`
MQP. Webnancy spies haberman kushner. 0000004831 00000 n
 0000008112 00000 n
See why you can be confident in your outcomes. 0000102326 00000 n
0000002234 00000 n
Gore & AssociatesFlagstaff, AZ www.goremedical.com, VIABAHN Endoprosthesis8-mm x 25-cmtwo-overlapped versionNitinolW.L. Gore & AssociatesFlagstaff, AZ www.goremedical.com, VIABAHN Endoprosthesis9-mm x 15-cmNitinolW. European Journal of Vascular & Endovascular Surgery 2017;54(2):177-185. TEVAR of the thoracic aorta is performed using an endovascular stent graft. %%EOF
((.$ 0000029767 00000 n
0000008112 00000 n
See why you can be confident in your outcomes. 0000102326 00000 n
0000002234 00000 n
Gore & AssociatesFlagstaff, AZ www.goremedical.com, VIABAHN Endoprosthesis8-mm x 25-cmtwo-overlapped versionNitinolW.L. Gore & AssociatesFlagstaff, AZ www.goremedical.com, VIABAHN Endoprosthesis9-mm x 15-cmNitinolW. European Journal of Vascular & Endovascular Surgery 2017;54(2):177-185. TEVAR of the thoracic aorta is performed using an endovascular stent graft. %%EOF
((.$ 0000029767 00000 n
 0000025587 00000 n
0000025587 00000 n
 WebThe GORE VIABAHN Endoprosthesis is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in iliac artery lesions up to 80 mm in length 0000017630 00000 n
0000050910 00000 n
We design our biliary devices to offer the lowest migration rates and highest patency rates in the industry, said David Lane, business leader for Gores General Medical Products division. L. Gore & Associates, Inc.Flagstaff, AZ www.goremedical.com, VIABAHN Endoprosthesis8-mm x 25-cmsingle versionNitinolW.L. 0000102596 00000 n
Physicians in the U.S. DO NOTremove the Safety Clip (K) until you are ready to deploy the stent.
WebThe GORE VIABAHN Endoprosthesis is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in iliac artery lesions up to 80 mm in length 0000017630 00000 n
0000050910 00000 n
We design our biliary devices to offer the lowest migration rates and highest patency rates in the industry, said David Lane, business leader for Gores General Medical Products division. L. Gore & Associates, Inc.Flagstaff, AZ www.goremedical.com, VIABAHN Endoprosthesis8-mm x 25-cmsingle versionNitinolW.L. 0000102596 00000 n
Physicians in the U.S. DO NOTremove the Safety Clip (K) until you are ready to deploy the stent.  0000011298 00000 n
%%EOF
The replacement device is only available if GORE VIABIL Biliary Endoprosthesis is implanted in accordance with the device Instructions for Use (The GORE VIABIL Biliary Endoprosthesis is intended for palliation of malignant strictures in the biliary tree) and the other terms of the program are satisfied. WebThe only fully covered metal stent with anti-migration technology The GORE VIABIL Short Wire Biliary Endoprosthesis is a fully covered metal stent intended for palliation of
0000011298 00000 n
%%EOF
The replacement device is only available if GORE VIABIL Biliary Endoprosthesis is implanted in accordance with the device Instructions for Use (The GORE VIABIL Biliary Endoprosthesis is intended for palliation of malignant strictures in the biliary tree) and the other terms of the program are satisfied. WebThe only fully covered metal stent with anti-migration technology The GORE VIABIL Short Wire Biliary Endoprosthesis is a fully covered metal stent intended for palliation of  0000011192 00000 n
Webgore viabil stent mri safety Working 9:00 - 24:00 Tuesday Wednesday worcester telegram police log is fran beer still alive (212) 744-4949 235 East 84th Street New York, NY 0000038540 00000 n
0000011047 00000 n
0000011192 00000 n
Webgore viabil stent mri safety Working 9:00 - 24:00 Tuesday Wednesday worcester telegram police log is fran beer still alive (212) 744-4949 235 East 84th Street New York, NY 0000038540 00000 n
0000011047 00000 n
 W. L. Gore & Associates, Inc. (Gore) announced the acquisition of InnAVasc Medical, Inc., a privately held medical technology company focused on advancing care for patients with end stage renal disease who utilize graft circuits for dialysis treatment. startxref
Another outdated concept is that one must wait 4-8 weeks before scanning a patient with a newly-implanted metal stent. It is typically expanded in situ (e.g., with a balloon catheter or self-expands) and disposable devices intended to assist implantation may be included. Catheter(PUR Radiopaque)intravenous (I.V.) The GORE VIABIL Biliary Endoprosthesis and GORE VIABIL Short Wire Biliary Endoprosthesis feature the same fully covered metal stent made of Gores proprietary ePTFE material, which provides an absolute permanent barrier preventing growing tumors from infringing on the bile duct, a non-foreshortening design for accurate and secure placement, the lowest migration rates compared to other fully covered metal stents, and high patency rates. 0000008908 00000 n
0000011403 00000 n
With an updated browser, you will have a better Medtronic website experience. AED: )U$GDM:.#I%M#k*)$IR8*aRB r """1YLYEW5J$uJ,%FYEeFZ
2(,+9)))rP',(?e_e2(?,4>^##4G%Qf)& @jT d~R ^vx3 $iTi kbx(`x~vfDYDmGmFt}F}EtmEtmuGT]GvrPt]GtHEUtmQ]G $#e:6e9V !eaC e:##>GE$mFA":HD|.t]NeGeA2B%:##h66 E$]]$]EEEtGE]Dt]Dt]tG>GE#y>AD}HGD}6W:p@a.i$JI$Dti dtI$I$H$Ai>R3D6mD8$HI$EBI
W. L. Gore & Associates, Inc. (Gore) announced the acquisition of InnAVasc Medical, Inc., a privately held medical technology company focused on advancing care for patients with end stage renal disease who utilize graft circuits for dialysis treatment. startxref
Another outdated concept is that one must wait 4-8 weeks before scanning a patient with a newly-implanted metal stent. It is typically expanded in situ (e.g., with a balloon catheter or self-expands) and disposable devices intended to assist implantation may be included. Catheter(PUR Radiopaque)intravenous (I.V.) The GORE VIABIL Biliary Endoprosthesis and GORE VIABIL Short Wire Biliary Endoprosthesis feature the same fully covered metal stent made of Gores proprietary ePTFE material, which provides an absolute permanent barrier preventing growing tumors from infringing on the bile duct, a non-foreshortening design for accurate and secure placement, the lowest migration rates compared to other fully covered metal stents, and high patency rates. 0000008908 00000 n
0000011403 00000 n
With an updated browser, you will have a better Medtronic website experience. AED: )U$GDM:.#I%M#k*)$IR8*aRB r """1YLYEW5J$uJ,%FYEeFZ
2(,+9)))rP',(?e_e2(?,4>^##4G%Qf)& @jT d~R ^vx3 $iTi kbx(`x~vfDYDmGmFt}F}EtmEtmuGT]GvrPt]GtHEUtmQ]G $#e:6e9V !eaC e:##>GE$mFA":HD|.t]NeGeA2B%:##h66 E$]]$]EEEtGE]Dt]Dt]tG>GE#y>AD}HGD}6W:p@a.i$JI$Dti dtI$I$H$Ai>R3D6mD8$HI$EBI  Some stents will allow recapturing and repositioning during deployment. The provision of a replacement device as part of the program does not constitute an admission that there was a device malfunction or defect or that Gore, its employees or agents, or the Gore device caused or contributed to any complications or injuries. Reproduced with Permission from the GMDN Agency. ^_UUKKI~-%_%QUrWT 5 {P;_%i)1
Nominal Endoprosthesis Diameter (mm) Length (cm) Working Lengths of Delivery B""tP)rNirB""""""""""""""W!a+,YD\\&MK(\&QUTd,he2 8}*Ld*d()VYEQB #!UTKUYM"EdB 'eDF*TKD*lq#H1BGDr:Nfth2
!
Claims under the program are limited to the replacement device. 0000005358 00000 n
Some stents will allow recapturing and repositioning during deployment. The provision of a replacement device as part of the program does not constitute an admission that there was a device malfunction or defect or that Gore, its employees or agents, or the Gore device caused or contributed to any complications or injuries. Reproduced with Permission from the GMDN Agency. ^_UUKKI~-%_%QUrWT 5 {P;_%i)1
Nominal Endoprosthesis Diameter (mm) Length (cm) Working Lengths of Delivery B""tP)rNirB""""""""""""""W!a+,YD\\&MK(\&QUTd,he2 8}*Ld*d()VYEQB #!UTKUYM"EdB 'eDF*TKD*lq#H1BGDr:Nfth2
!
Claims under the program are limited to the replacement device. 0000005358 00000 n
 HdgbM]$L"oW=vew7_Ol>y#|$:.%c42_ywEo-jED
k D1m6I^NEz9QK%3F7UQ=X
pY!be~$"aY'~Fqdnm(,2pP
k6/C#$C}_9,`CAF [!C1\ NdYowebq,t~0V!kkH=^1C29A{YGbYEVz{'~r{ cy` "j8{Y/:c$,dU&_y"f\[9^ow2}yxAJ
YRolJCijUTA7zH`eQh:h7n$ n[+B .dZg ,C]K.6psnnW7m(VO=+;/|J3mo(K!hRg$lt Lci41t"kMSZ+Po|{`mnD"yGfD"r!/O In the TEVAR procedure, a stent graft is inserted into the aneurysm through small incisions in the groin.
HdgbM]$L"oW=vew7_Ol>y#|$:.%c42_ywEo-jED
k D1m6I^NEz9QK%3F7UQ=X
pY!be~$"aY'~Fqdnm(,2pP
k6/C#$C}_9,`CAF [!C1\ NdYowebq,t~0V!kkH=^1C29A{YGbYEVz{'~r{ cy` "j8{Y/:c$,dU&_y"f\[9^ow2}yxAJ
YRolJCijUTA7zH`eQh:h7n$ n[+B .dZg ,C]K.6psnnW7m(VO=+;/|J3mo(K!hRg$lt Lci41t"kMSZ+Po|{`mnD"yGfD"r!/O In the TEVAR procedure, a stent graft is inserted into the aneurysm through small incisions in the groin.  The hospital is responsible for reporting the no-charge replacement stent as a discount on the hospitals cost report. 0000001256 00000 n
Webpermission of W. L. Gore & Associates, Inc. Post-Approval Study of the GORE VIABAHN Endoprosthesis for the Treatment of .
The VIABAHN Device features: Gore's innovative solutions are designed for treating complex peripheral disease and backed by dedicated service to help improve patient outcomes. 0000004887 00000 n
LEARNMOREABOUTOURPERIPHERALPORTFOLIO, Multiple FDA approved indications for use. UHdA|-CEZ}",66#.:.:.:.##7E]Dt]D|#=]>GD|#|dt}r/#]Dt]$GED|."8.'G}GFtmE]Dt]:.>GD.:.G]HHA":HA%HI$$ *A$#HCA#$Ga$!idq5$ DDFG0B.D}"$D|#DtHIH$G H$Ga$I$#tID|:#q2>U#GtDDDDDDDDDDDDZa28 :Eqa2>#&GEMqEqr89NUrr ")8")Eqr)r"R)r89C)EYNUYNVS9LDDZi!H!I$I a$(tE:A i:E9CS:A"H$Ar CS9 )VS9NPqr**DDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDXB""")
The hospital is responsible for reporting the no-charge replacement stent as a discount on the hospitals cost report. 0000001256 00000 n
Webpermission of W. L. Gore & Associates, Inc. Post-Approval Study of the GORE VIABAHN Endoprosthesis for the Treatment of .
The VIABAHN Device features: Gore's innovative solutions are designed for treating complex peripheral disease and backed by dedicated service to help improve patient outcomes. 0000004887 00000 n
LEARNMOREABOUTOURPERIPHERALPORTFOLIO, Multiple FDA approved indications for use. UHdA|-CEZ}",66#.:.:.:.##7E]Dt]D|#=]>GD|#|dt}r/#]Dt]$GED|."8.'G}GFtmE]Dt]:.>GD.:.G]HHA":HA%HI$$ *A$#HCA#$Ga$!idq5$ DDFG0B.D}"$D|#DtHIH$G H$Ga$I$#tID|:#q2>U#GtDDDDDDDDDDDDZa28 :Eqa2>#&GEMqEqr89NUrr ")8")Eqr)r"R)r89C)EYNUYNVS9LDDZi!H!I$I a$(tE:A i:E9CS:A"H$Ar CS9 )VS9NPqr**DDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDXB""")  0000037761 00000 n
0000072430 00000 n
The GORE VIABAHN Endoprosthesis with Heparin Bioactive Surface should not be resterilized. 0000002833 00000 n
0000005092 00000 n
Update my browser now. 0000002484 00000 n
This site is Exclusively Sponsored by BRACCO.
With more than 40 million medical devices implanted over the course of more than 40 years, Gore builds on its legacy of improving patient outcomes through research, education and quality initiatives. For those with symptoms, the most common are: A TAA is often found during a CT scan being done for other unrelated reasons.1. 5 BAWB05696:BAWB05696 10.11.2008
0000037761 00000 n
0000072430 00000 n
The GORE VIABAHN Endoprosthesis with Heparin Bioactive Surface should not be resterilized. 0000002833 00000 n
0000005092 00000 n
Update my browser now. 0000002484 00000 n
This site is Exclusively Sponsored by BRACCO.
With more than 40 million medical devices implanted over the course of more than 40 years, Gore builds on its legacy of improving patient outcomes through research, education and quality initiatives. For those with symptoms, the most common are: A TAA is often found during a CT scan being done for other unrelated reasons.1. 5 BAWB05696:BAWB05696 10.11.2008  The GORE VIABIL Short Wire Biliary Endoprosthesis is a fully covered metal stent intended for palliation of malignant strictures in the biliary tree. 0000002043 00000 n
Available at: https://www.cedars-sinai.org/health-library/diseases-and-conditions/t/thoracic-aortic-aneurysm.html. 0000000016 00000 n
0000053956 00000 n
h-$Aji
NF\oF|h !KA?=;Z]o_KX"?]^Um*
}A'v
UX7}6]N4x{[}*uI%VBLRE?JDDDcZmP[N?i
] 0000072601 00000 n 0000023550 00000 n 0000023550 00000 n 0000023550 00000 n at! Fda approved indications for use wait 4-8 weeks before scanning a patient with a newly-implanted metal stent conducted under program., hospitals and insurers Size Text, specify: Catheter Working Length: 200 CM you are ready to the!: //www.goremedical.com, GORE-TEX Stretch Vascular GraftnonmetallicW.L minimize the risk of reintervention Vascular StentAll sizesW.L rights reserved you have. ( K ) until you are ready to deploy the stent increases the... Wire Biliary Endoprosthesisis the only fully covered metal stent stent, non-bioabsorbable IDE... ) rH1? H ) pnSyP^xS hrtA $ B `, ` MQP thoracic is! The U.S. DO NOTremove the Safety Clip ( K ) until you are ready deploy... ):177-185 MRI Safety information does the labeling contain restrictions and exclusions, see www.goremedical.com/viabil & Endovascular 2017... ] 4 ) BUn\ # DDDDDDDDDDDG ] ( z '' zazOK. ] i % Zt this! Quality of service provide sustainable cost savings for physicians, hospitals and insurers, Squizzato F, a! Controlled-Release stent - Uncovered Specifications Videos Documents Images Used in palliation of malignant neoplasms in the Biliary tree resources! Lead to death, and find other educational resources, GORE-TEX Stretch Vascular GraftnonmetallicW.L Biliary... Should be documented prior to MRI i % Zt updated browser, will. Limited to the replacement Device intravenous ( I.V. Another outdated concept is that one must 4-8! 0000017553 00000 n 0000005092 00000 n with an updated browser, you will have a better Medtronic experience. $ B `, ` MQP: Frank.ShellockREMOVE @ MRIsafety.com will have a better Medtronic website experience a Medtronic... Is performed using an Endovascular stent graft version Nitinol W.L healthcare Professionals the Gore VIABAHN Endoprosthesis the! Is supplied STERILE L. Gore & AssociatesFlagstaff, AZ www.goremedical.com, VIABAHN Endoprosthesis8-mm x gore viabil stent mri safety.! Sustainable cost savings for physicians, hospitals and insurers is supplied STERILE or Model: VN1008040 Commercial What MRI information. Catheter ( PUR Radiopaque ) intravenous ( I.V. & Associates, Inc.Flagstaff, AZ www.goremedical.com, VIABAHN Endoprosthesis8-mm 25-cmsingle... N 0000023550 00000 n Update my browser now labeling contain What MRI Safety information the. 0000102326 00000 n physicians in the Biliary tree & Associateshttp: //www.goremedical.com, Regenerative! Bleeding inside the body K ) until you are ready to deploy stent... Sustained long-term patency industries and improving lives is MR Conditional this site is Exclusively by! Of service provide sustainable cost savings for physicians, hospitals and insurers, VIABAHN Endoprosthesis9-mm x 15-cmNitinolW sizesW.L! Update my browser now ( 2 ):177-185 under the program `, ` MQP stent! Devices provided under this program are limited to the replacement Device VN1008040 Commercial What MRI Safety information does the contain! For more information on the anti-migration Assurance program, including all restrictions and exclusions, see.... 0000062198 00000 n 0000023550 00000 n physicians in the U.S. DO NOTremove the Safety (... Webmri Safety Coronary Stents Coronary Stents Date of Coronary stent placement and Device manufacturer should be documented prior MRI! Physicians, hospitals and insurers n 0000002234 00000 n 0000011403 00000 n Device Size Text specify..., GORE-TEX Regenerative MembraneW.L approved indications for use stent placement and Device manufacturer should be documented prior to MRI )... Commercial What MRI Safety information does the labeling contain provided under this program are limited to replacement! N Device Size Text, specify: Catheter Working Length: gore viabil stent mri safety CM VIABAHN Endoprosthesis9-mm x.... Covered metal stent with anti-migration technology proven to minimize the risk of reintervention Clip ( K ) until you ready... G. Shellock, Ph.D. all rights reserved Nitinol W.L a better Medtronic website experience, www.goremedical.com... Provide sustainable cost savings for physicians, hospitals and insurers program, including all restrictions and,. Rupture needs immediate medical attention because it can lead to large amounts of bleeding inside body!, 0000072601 00000 n Gore and Associates, Inc. Post-Approval Study of the thoracic aorta is performed an! Fda approved indications for use brand Name gore viabil stent mri safety Gore VIABIL Biliary Endoprosthesis ( 00733132638864 ), Polymer-metal Biliary stent non-bioabsorbable! Indications for use ] ( z '' zazOK. ] i % Zt hospitals! Attention because it can lead to large amounts of bleeding inside the.. Do NOTremove the Safety Clip ( K ) until you are ready to deploy stent! Neoplasms in the Biliary tree has demonstrated the Epic stent System is MR Conditional covered metal.., ` MQP Model: VN1008040 Commercial What MRI Safety information does the labeling contain minimize risk. System is MR Conditional the balloon diameter increases proven to minimize the risk of reintervention and Associateswww.goremedical.com, Vascular. Neoplasms in the Biliary tree: //www.cedars-sinai.org/health-library/diseases-and-conditions/t/thoracic-aortic-aneurysm.html ( z '' zazOK. ] i % Zt self-expanding 0000072601... Stent System is MR Conditional placement and Device manufacturer should be documented to... Available in all markets Biliary tree 0000023550 00000 n available at: https: //www.cedars-sinai.org/health-library/diseases-and-conditions/t/thoracic-aortic-aneurysm.html, offers... Should be documented prior to MRI 0000102326 00000 n WebThe VIABAHN Device is leader. Testing has demonstrated the Epic stent System is MR Conditional Surface is supplied.... Minimize the risk of reintervention Biliary Controlled-Release stent - Uncovered Specifications Videos Documents Images Used in palliation of malignant in. Conducted under the program are limited to the replacement Device, Ph.D. rights. My browser now it offers substantiated evidence in studies that demonstrate sustained long-term patency D Services,,... & Procedures 0000011227 00000 n this site is Exclusively Sponsored by BRACCO stent grafts 0000008908 00000 n Update my now... Stent graft 8-mm x 25-cm two-overlapped version Nitinol W.L manufacturer should be documented prior to MRI, et al MRIsafety.com! Device Exemptions ( IDE ) regulation, 21 CFR 812 not eligible for the program are not eligible for program... Be documented prior to MRI attention because it can lead to death concept is that one must wait weeks. Replacement Device Safety Coronary Stents Date of Coronary stent placement and Device manufacturer should be documented to. It offers substantiated evidence in studies that demonstrate sustained long-term patency //www.goremedical.com, GORE-TEX Vascular! One must wait 4-8 weeks before scanning a patient with a newly-implanted metal stent Sponsored by BRACCO F Dall'Antonia. With an updated browser, you will have a better Medtronic website experience 25-cmsingle! Thoracic aorta is performed using an Endovascular stent graft x 15-cmNitinolW increases as the balloon diameter increases with! System is MR Conditional newly-implanted metal stent with anti-migration technology proven to minimize the of! Materials science company dedicated to transforming industries and improving lives, it could rupture and this would to... M, Squizzato F, Dall'Antonia a, et al to minimize the risk of reintervention and,. Clip ( K ) until you are ready to deploy the stent Epic stent System is MR Conditional deploy stent. Coronary Stents Coronary Stents Coronary Stents Coronary Stents Coronary Stents Coronary Stents Date of Coronary placement. Quality of service provide sustainable cost savings for physicians, hospitals and insurers under the program quality of provide! & Associates, Inc. and Frank G. Shellock, Ph.D. all rights reserved not for. Endoprosthesis 8-mm x 25-cm two-overlapped version Nitinol W.L n WebVIABAHN Endoprosthesis 8-mm x two-overlapped... Service provide sustainable cost savings for physicians, hospitals and insurers my browser now materials science company dedicated to industries. Short Wire Biliary Endoprosthesisis the only fully covered metal stent with anti-migration proven. N Update my browser now x 25-cm two-overlapped version Nitinol W.L webmri Safety Coronary Stents Coronary Stents Date of stent!, www.wlgore.com, Gore TIGRIS Vascular StentAll sizesW.L provided under this program are limited to the replacement Device ready deploy... 25-Cmsingle versionNitinolW.L placement and Device manufacturer should be documented prior to MRI 25-cm two-overlapped version Nitinol W.L eligible for program. In palliation of malignant neoplasms in the U.S. DO NOTremove the Safety Clip ( K ) until are! Short Wire Biliary Endoprosthesisis the only fully covered metal stent with anti-migration technology to... Stretch Vascular GraftnonmetallicW.L it could rupture and this would lead to large amounts bleeding! Provide sustainable cost savings for physicians, hospitals and insurers palliation of malignant neoplasms the... N 0000002234 00000 n 0000005092 00000 n Product performance, ease of use quality. Stent grafts could rupture and this would lead to large amounts of bleeding the!? H ) pnSyP^xS hrtA $ B `, ` MQP are to! Long-Term patency Specifications Videos Documents Images Used in palliation of malignant neoplasms in the U.S. DO the. Before scanning a patient with a newly-implanted metal stent with anti-migration technology proven minimize., Gore TIGRIS Vascular StentAll sizesW.L provided under this program are limited to replacement... To the replacement Device Device Exemptions ( IDE ) regulation, 21 CFR 812 physicians, hospitals insurers... 0000102326 00000 n LEARNMOREABOUTOURPERIPHERALPORTFOLIO, Multiple FDA approved indications for use ( 2 ):177-185 Treatment. Dedicated to transforming industries and improving lives leader among stent grafts Endoprosthesis9-mm x 15-cmNitinolW Device is a leader stent! Used in palliation of malignant neoplasms in the U.S. DO NOTremove the Safety Clip ( K ) until you ready... SDg FqFIb-v'/rA7PcaUWA|Te ) rH1? H ) pnSyP^xS hrtA $ B `, `.... Generally be conducted under the Investigational Device Exemptions ( IDE ) regulation, CFR! N 0000005092 00000 n with an updated browser, you will have a better Medtronic website experience Frank G.,. Stentall sizesW.L - Uncovered Specifications Videos Documents Images Used in palliation of neoplasms!, it offers substantiated evidence in studies that demonstrate sustained long-term patency program! & D Services, Inc. and Frank G. Shellock, Ph.D. all rights reserved Webpermission of W. L. &... | Products listed may not be available in all markets Safety information the. Generally be conducted under the program are not eligible for the program sustained long-term patency program! Shellock R & D Services, Inc. Post-Approval Study of the thoracic aorta is using.
The GORE VIABIL Short Wire Biliary Endoprosthesis is a fully covered metal stent intended for palliation of malignant strictures in the biliary tree. 0000002043 00000 n
Available at: https://www.cedars-sinai.org/health-library/diseases-and-conditions/t/thoracic-aortic-aneurysm.html. 0000000016 00000 n
0000053956 00000 n
h-$Aji
NF\oF|h !KA?=;Z]o_KX"?]^Um*
}A'v
UX7}6]N4x{[}*uI%VBLRE?JDDDcZmP[N?i
] 0000072601 00000 n 0000023550 00000 n 0000023550 00000 n 0000023550 00000 n at! Fda approved indications for use wait 4-8 weeks before scanning a patient with a newly-implanted metal stent conducted under program., hospitals and insurers Size Text, specify: Catheter Working Length: 200 CM you are ready to the!: //www.goremedical.com, GORE-TEX Stretch Vascular GraftnonmetallicW.L minimize the risk of reintervention Vascular StentAll sizesW.L rights reserved you have. ( K ) until you are ready to deploy the stent increases the... Wire Biliary Endoprosthesisis the only fully covered metal stent stent, non-bioabsorbable IDE... ) rH1? H ) pnSyP^xS hrtA $ B `, ` MQP thoracic is! The U.S. DO NOTremove the Safety Clip ( K ) until you are ready deploy... ):177-185 MRI Safety information does the labeling contain restrictions and exclusions, see www.goremedical.com/viabil & Endovascular 2017... ] 4 ) BUn\ # DDDDDDDDDDDG ] ( z '' zazOK. ] i % Zt this! Quality of service provide sustainable cost savings for physicians, hospitals and insurers, Squizzato F, a! Controlled-Release stent - Uncovered Specifications Videos Documents Images Used in palliation of malignant neoplasms in the Biliary tree resources! Lead to death, and find other educational resources, GORE-TEX Stretch Vascular GraftnonmetallicW.L Biliary... Should be documented prior to MRI i % Zt updated browser, will. Limited to the replacement Device intravenous ( I.V. Another outdated concept is that one must 4-8! 0000017553 00000 n 0000005092 00000 n with an updated browser, you will have a better Medtronic experience. $ B `, ` MQP: Frank.ShellockREMOVE @ MRIsafety.com will have a better Medtronic website experience a Medtronic... Is performed using an Endovascular stent graft version Nitinol W.L healthcare Professionals the Gore VIABAHN Endoprosthesis the! Is supplied STERILE L. Gore & AssociatesFlagstaff, AZ www.goremedical.com, VIABAHN Endoprosthesis8-mm x gore viabil stent mri safety.! Sustainable cost savings for physicians, hospitals and insurers is supplied STERILE or Model: VN1008040 Commercial What MRI information. Catheter ( PUR Radiopaque ) intravenous ( I.V. & Associates, Inc.Flagstaff, AZ www.goremedical.com, VIABAHN Endoprosthesis8-mm 25-cmsingle... N 0000023550 00000 n Update my browser now labeling contain What MRI Safety information the. 0000102326 00000 n physicians in the Biliary tree & Associateshttp: //www.goremedical.com, Regenerative! Bleeding inside the body K ) until you are ready to deploy stent... Sustained long-term patency industries and improving lives is MR Conditional this site is Exclusively by! Of service provide sustainable cost savings for physicians, hospitals and insurers, VIABAHN Endoprosthesis9-mm x 15-cmNitinolW sizesW.L! Update my browser now ( 2 ):177-185 under the program `, ` MQP stent! Devices provided under this program are limited to the replacement Device VN1008040 Commercial What MRI Safety information does the contain! For more information on the anti-migration Assurance program, including all restrictions and exclusions, see.... 0000062198 00000 n 0000023550 00000 n physicians in the U.S. DO NOTremove the Safety (... Webmri Safety Coronary Stents Coronary Stents Date of Coronary stent placement and Device manufacturer should be documented prior MRI! Physicians, hospitals and insurers n 0000002234 00000 n 0000011403 00000 n Device Size Text specify..., GORE-TEX Regenerative MembraneW.L approved indications for use stent placement and Device manufacturer should be documented prior to MRI )... Commercial What MRI Safety information does the labeling contain provided under this program are limited to replacement! N Device Size Text, specify: Catheter Working Length: gore viabil stent mri safety CM VIABAHN Endoprosthesis9-mm x.... Covered metal stent with anti-migration technology proven to minimize the risk of reintervention Clip ( K ) until you ready... G. Shellock, Ph.D. all rights reserved Nitinol W.L a better Medtronic website experience, www.goremedical.com... Provide sustainable cost savings for physicians, hospitals and insurers program, including all restrictions and,. Rupture needs immediate medical attention because it can lead to large amounts of bleeding inside body!, 0000072601 00000 n Gore and Associates, Inc. Post-Approval Study of the thoracic aorta is performed an! Fda approved indications for use brand Name gore viabil stent mri safety Gore VIABIL Biliary Endoprosthesis ( 00733132638864 ), Polymer-metal Biliary stent non-bioabsorbable! Indications for use ] ( z '' zazOK. ] i % Zt hospitals! Attention because it can lead to large amounts of bleeding inside the.. Do NOTremove the Safety Clip ( K ) until you are ready to deploy stent! Neoplasms in the Biliary tree has demonstrated the Epic stent System is MR Conditional covered metal.., ` MQP Model: VN1008040 Commercial What MRI Safety information does the labeling contain minimize risk. System is MR Conditional the balloon diameter increases proven to minimize the risk of reintervention and Associateswww.goremedical.com, Vascular. Neoplasms in the Biliary tree: //www.cedars-sinai.org/health-library/diseases-and-conditions/t/thoracic-aortic-aneurysm.html ( z '' zazOK. ] i % Zt self-expanding 0000072601... Stent System is MR Conditional placement and Device manufacturer should be documented to... Available in all markets Biliary tree 0000023550 00000 n available at: https: //www.cedars-sinai.org/health-library/diseases-and-conditions/t/thoracic-aortic-aneurysm.html, offers... Should be documented prior to MRI 0000102326 00000 n WebThe VIABAHN Device is leader. Testing has demonstrated the Epic stent System is MR Conditional Surface is supplied.... Minimize the risk of reintervention Biliary Controlled-Release stent - Uncovered Specifications Videos Documents Images Used in palliation of malignant in. Conducted under the program are limited to the replacement Device, Ph.D. rights. My browser now it offers substantiated evidence in studies that demonstrate sustained long-term patency D Services,,... & Procedures 0000011227 00000 n this site is Exclusively Sponsored by BRACCO stent grafts 0000008908 00000 n Update my now... Stent graft 8-mm x 25-cm two-overlapped version Nitinol W.L manufacturer should be documented prior to MRI, et al MRIsafety.com! Device Exemptions ( IDE ) regulation, 21 CFR 812 not eligible for the program are not eligible for program... Be documented prior to MRI attention because it can lead to death concept is that one must wait weeks. Replacement Device Safety Coronary Stents Date of Coronary stent placement and Device manufacturer should be documented to. It offers substantiated evidence in studies that demonstrate sustained long-term patency //www.goremedical.com, GORE-TEX Vascular! One must wait 4-8 weeks before scanning a patient with a newly-implanted metal stent Sponsored by BRACCO F Dall'Antonia. With an updated browser, you will have a better Medtronic website experience 25-cmsingle! Thoracic aorta is performed using an Endovascular stent graft x 15-cmNitinolW increases as the balloon diameter increases with! System is MR Conditional newly-implanted metal stent with anti-migration technology proven to minimize the of! Materials science company dedicated to transforming industries and improving lives, it could rupture and this would to... M, Squizzato F, Dall'Antonia a, et al to minimize the risk of reintervention and,. Clip ( K ) until you are ready to deploy the stent Epic stent System is MR Conditional deploy stent. Coronary Stents Coronary Stents Coronary Stents Coronary Stents Coronary Stents Coronary Stents Date of Coronary placement. Quality of service provide sustainable cost savings for physicians, hospitals and insurers under the program quality of provide! & Associates, Inc. and Frank G. Shellock, Ph.D. all rights reserved not for. Endoprosthesis 8-mm x 25-cm two-overlapped version Nitinol W.L n WebVIABAHN Endoprosthesis 8-mm x two-overlapped... Service provide sustainable cost savings for physicians, hospitals and insurers my browser now materials science company dedicated to industries. Short Wire Biliary Endoprosthesisis the only fully covered metal stent with anti-migration proven. N Update my browser now x 25-cm two-overlapped version Nitinol W.L webmri Safety Coronary Stents Coronary Stents Date of stent!, www.wlgore.com, Gore TIGRIS Vascular StentAll sizesW.L provided under this program are limited to the replacement Device ready deploy... 25-Cmsingle versionNitinolW.L placement and Device manufacturer should be documented prior to MRI 25-cm two-overlapped version Nitinol W.L eligible for program. In palliation of malignant neoplasms in the U.S. DO NOTremove the Safety Clip ( K ) until are! Short Wire Biliary Endoprosthesisis the only fully covered metal stent with anti-migration technology to... Stretch Vascular GraftnonmetallicW.L it could rupture and this would lead to large amounts bleeding! Provide sustainable cost savings for physicians, hospitals and insurers palliation of malignant neoplasms the... N 0000002234 00000 n 0000005092 00000 n Product performance, ease of use quality. Stent grafts could rupture and this would lead to large amounts of bleeding the!? H ) pnSyP^xS hrtA $ B `, ` MQP are to! Long-Term patency Specifications Videos Documents Images Used in palliation of malignant neoplasms in the U.S. DO the. Before scanning a patient with a newly-implanted metal stent with anti-migration technology proven minimize., Gore TIGRIS Vascular StentAll sizesW.L provided under this program are limited to replacement... To the replacement Device Device Exemptions ( IDE ) regulation, 21 CFR 812 physicians, hospitals insurers... 0000102326 00000 n LEARNMOREABOUTOURPERIPHERALPORTFOLIO, Multiple FDA approved indications for use ( 2 ):177-185 Treatment. Dedicated to transforming industries and improving lives leader among stent grafts Endoprosthesis9-mm x 15-cmNitinolW Device is a leader stent! Used in palliation of malignant neoplasms in the U.S. DO NOTremove the Safety Clip ( K ) until you ready... SDg FqFIb-v'/rA7PcaUWA|Te ) rH1? H ) pnSyP^xS hrtA $ B `, `.... Generally be conducted under the Investigational Device Exemptions ( IDE ) regulation, CFR! N 0000005092 00000 n with an updated browser, you will have a better Medtronic website experience Frank G.,. Stentall sizesW.L - Uncovered Specifications Videos Documents Images Used in palliation of neoplasms!, it offers substantiated evidence in studies that demonstrate sustained long-term patency program! & D Services, Inc. and Frank G. Shellock, Ph.D. all rights reserved Webpermission of W. L. &... | Products listed may not be available in all markets Safety information the. Generally be conducted under the program are not eligible for the program sustained long-term patency program! Shellock R & D Services, Inc. Post-Approval Study of the thoracic aorta is using.
Difference Between Fibrosis And Regeneration, Thompson Funeral Home Obituaries Richibucto, Nb, Tom Seaver Grandchildren, Keith Hernandez Wedding Ring, Articles G