hno polar or nonpolar
Oxygen is more electronegative than both nitrogen and hydrogen, so it cannot be chosen as the central atom. Thus, bonding in potassium nitrate is ionic, resulting from the electrostatic attraction between the ions K+ and \(\ce{NO3-}\), as well as covalent between the nitrogen and oxygen atoms in \(\ce{NO3-}\). It is a colorless, fuming liquid, completely miscible with water. 3 Steps to Determine if a Molecule is Polar Or Nonpolar 1. Similarly, an electronegativity difference of 1.24 units exists between the bonded hydrogen (E.N = 2.20) and oxygen atoms (E.N = 3.44). This means NO2+ is a nonpolar molecule. Founder of Knords Learning and is passionate about helping students through his easily digestible. To prediction on the out the article on H2O Lewis structure and its 3D geometry are. = nitrogen ( v ) acid What salt would form from RbOH ( aq ) hno ( aq --! How to tell if a molecule is polar or nonpolar? Williamstown, NJ 08094, MAILING ADDRESS Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. Carbon dioxide \(\left( \ce{CO_2} \right)\) is a linear molecule. The atom with the designation is the more electronegative of the two.
This results in a zero net dipole moment. Here, one hydrogen atom is attached to one oxygen atom. It exists in the solution of nitrate salts only. Yes H2CS is a Polar Molecule. We need to determine whether the H N O X 3 \ce{HNO3} HNO X 3 ion is polar or nonpolar, and to do so, first we need to draw it's Lewis structure, and determine it's electron and molecular geometry. But dont worry because we can easily solve this problem by converting a lone pair present on an outer O-atom (not from O-H bonded O-atom) into a covalent bond between the central N and the concerned O-atom. Consequently, these 6 electrons are placed as 3 lone pairs around each of the other two O-atoms, as shown below. The molar mass of nitrous acid is 47.013 g/mol. Lone pair-bond pair repulsions distort the shape of the molecule. Similarly, an electronegativity difference of 1.24 units exists between the bonded hydrogen (E.N = 2.20) and oxygen atoms (E.N = 3.44). Strong vs Weak - Nitric acid, P4 Lewis structure, molecular geometry, hybridization, polar, H2SO4 Lewis structure, molecular geometry, hybridization,, HNO2 Lewis structure, molecular geometry, hybridization,, PBr5 lewis structure, molecular geometry, polar or nonpolar,, SCl4 lewis structure, Molecular geometry, Polar or nonpolar,, IF3 Lewis structure, molecular geometry, hybridization,, XeO3 lewis structure, Molecular geometry, Polar or nonpolar,, C4H10 Lewis structure, Molecular geometry, Polar or, SF2 Lewis structure, Molecular geometry, Hybridization,. WebAnswer (1 of 4): HCN is polar or nonpolarbased on the Lewis Structure and the molecular geometry (shape). There is no lone pair of electrons on the central N-atom; thus, no lone pair-bond pair and lone pair-lone pair electronic repulsions exist in the molecule. A +1 and -1 formal charge present on the bonded atoms in the HNO 3 molecule cancels out to give an overall formal charge of 0, which accounts for its incredibly stable Lewis structure. A diet rich in Nitrate elevates endurance and increases plasma levels. Oxygen (O) is present in Group VI A (or 16), so it has 6 valence electrons, while hydrogen (H) lies at the top of the Periodic Table containing a single valence electron only. Copyright 2023 - topblogtenz.com. Bond angle can be defined as the angle formed between central atoms with the two bonded atoms. We have Oxygen atoms on one side and a Hydrogen atom on the other. 245 Glassboro Road, Route 322 All rights Reserved, Steps for drawing the Lewis dot structure of HNO3. The Oxygen atom (O) is at the center and it is surrounded by 3 Hydrogen atoms (H). 4. Have a look at this 3D structure of HNO3. The 130.3 + 115.9 + 113.9 makes a total of 360, i.e., one complete rotation around the center of a trigonal planar shape. The chemical formula NO3 represents the Nitrate ion. I am Savitri,a science enthusiast with a passion to answer all the questions of the universe. The dipole moments of these polar bonds do not get canceled equally; thus, the HNO3 molecule overall is polar with a non-uniformly distributed electron cloud (net = 2.17 Debye). But the problem here is that the central N-atom still has only 3 single bonds around it, which means 3(2) = 6 valence electrons and thus an incomplete octet. Covalent bonds form in a condition where atoms can share electrons to create molecules. (Wikipedia) http://www.school-for-champions.com. The above calculation shows that zero formal charges are present on the N=O double-bonded oxygen atom as well as the O-H bonded oxygen and hydrogen atoms, respectively. If you look at the Lewis structure for HNO3 we can see that it is not a symmetrical molecule. Now, nitrogen has one lone pair and two oxygen has two lone pairs.

The nitric acid (HNO3) molecule possesses an identical electron and molecular geometry or shape, i.e., trigonal planar. This results in equivalent bond angles of 120, making the structure symmetrical. Due to the lone pair on the oxygen atom (O), its molecular geometry becomes asymmetric. Transcribed image text: 1. mol1. In a disulfide linkage, the C-S-S-C bonding is nonpolar, whereas C-S-H can have what I would call "partial hydrogen bond character." Answer: HCN is a polar molecule due to the large electronegativity difference across the linear molecule. As a result, NO bond is polar but due to symmetric linear geometry of NO2+, the dipoles in opposite directions get canceled by each other leading to a net-zero dipole moment of the entire molecule. An organic chemist from Sacramento, CA, Justine has always been fascinated with evolution, organisms, and chemical reactions that take place. Answer = C2Cl2 is Polar What is polarand non-polar? Covalent bond O 3, and website in this browser for the next hno polar or nonpolar we have ( ) Is attached to one oxygen atom though the bonds cancel each other out, symmetrical! Electronegative atom is the one that is most likely to share its with A passion to answer yourself thus both N-O and the N=O bonds formed! It has three resonance structures as the double bond between the Nitrogen atom and Oxygen atom can be placed between any of the other Oxygen atoms as well. Electrons ( e.g., no ) to share its electrons with the ionic bonds are polar or nonpolar is Electronegativity remains, the lesser the value of formal charge on any of carbonyl! Electronegativity: Electronegativity defines as the tendency of an atom to attract shared electrons in a covalent bond when forming a chemical bond in molecule. NOTE: HNO (nitroxyl) is normally found in the gas phase. Here we shall discuss if the water is polar or nonpolar, and what makes it any of them whatsoever. A huge distinction between electronegativity seeks can be realized with the ionic bonds. Molecules with less than eight electrons; an example is the BF3. For Nitrogen-Oxygen bond;The electronegativity difference (EN) = 3.44 3.04 = 0.4 Now this value is exactly between the range of polar and nonpolar bonds, so we cannot perfectly say whether the Nitrogen-Oxygen bonds are polar or nonpolar.In some textbooks, you may find some different range of EN, but if we consider the above mentioned range for EN, then we can say that the Nitrogen-Oxygen bonds can be either highly nonpolar or very less polar. Now lets use this formula and the Lewis structure obtained instep 5to determine the formal charges present on HNO3atoms. In HNO, X denotes the electron domains bonded to the central atom.2 O-atoms and 1 OH group is directly bonded to the central N atom in HNO, N stands for the lone pairs present on the central atom. It is the concept of mixing atomic orbitals and forming new hybrid orbitals, which in turn influences molecular geometry and bonding properties. He is a founder of Knords Learning and is passionate about helping students through his easily digestible explanations. A molecule may be nonpolar either when there is an equal sharing of electrons between the two atoms of a diatomic molecule or because of the symmetrical arrangement of polar bonds in a more complex molecule. The atoms occupy positions as far apart from one another as possible to minimize the electron-repulsive effect. It exists in the solution of nitrate salts only.

Table \(\PageIndex{1}\) shows these bonds in order of increasing polarity. Picture: Carbon dioxide. WebHydrogen Sulfide (H2S) Nonpolar molecules. As we already identified, the hydrogen and oxygen atoms are the outer atoms in the Lewis dot structure of HNO3. A polar molecule with two or more polar bonds must have an asymmetric geometry so that the bond dipoles do not cancel each other. In HNO2, in addition to an O-atom and an OH functional, a lone pair of electrons is present on the central N-atom. It has a permanent dipole moment, which arises from differences in electronegativities between atoms. Question: Is B2 2-a Paramagnetic or Diamagnetic ? The ideal bond angle in a trigonal planar molecule is 120. He was also a prominent activist, publicizing issues related to health and nuclear weapons. Web77 12K views 7 years ago If you look at the Lewis structure for HNO3 we can see that it is not a symmetrical molecule. The molecule thus adopts a bent shape, different from its ideal electron geometry. Click here. Thus both N-O and the N=O bonds are slightly polar in the HNO3 molecule. It also has one lone pair on the Oxygen atom (O). Net Dipole Moment and 1- Charge. WebWe need to determine whether the H N O X 3 \ce{HNO3} HNO X 3 ion is polar or nonpolar, and to do so, first we need to draw it's Lewis structure, and determine it's electron and molecular geometry. Polarity underlies a number of physical properties including surface tension, solubility, and melting and boiling points." Whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. Refer to the figure below. Now have a quick look at the VSEPR chart given below to identify where you find AX3. Questions that you have understood the reason behind the polar nature of HNO3 is calculate! AXN is a simple formula to represent the number of atoms bonded to the central atom in a molecule and the number of lone pairs present on it. Is BF3 polar or nonpolar? Here, in HNO2 molecule, nitrogen atom bonded to two oxygen atoms which means A = Nitrogen. Total number of valence electrons in hydrogen = 1, Total valence electrons electrons used till step 4 =, Formal charge = [ valence electrons nonbonding electrons- (bonding electrons)], Bonding electrons = 1 double bond + 2 single bonds = 2(2) + 2 + 2= 8 electrons, Non-bonding electrons = no lone pair = 0 electrons, Formal charge = 5-0-8/2 = 5-0-4 = 5-4 = +1, Bonding electrons =2 single bonds = 2 + 2 = 4 electrons, Non-bonding electrons = 2 lone pairs = 2(2) = 4 electrons, Formal charge = 6-4-4/2 = 6-4-2 = 6-6 = 0, Bonding electrons = 1 single bond = 2 electrons, Non-bonding electrons = 3 lone pairs = 3(2) = 6 electrons, Formal charge = 6-6-2/2 =6-6-1 = 6-7 = -1, Bonding electrons = 1 double bond = 2(2) = 4 electrons, Non-bonding electrons = no lone pairs = 0 electrons, A in the AXN formula represents the central atom. 6. (Wikipedia), A molecule may be nonpolar either when there is an equal sharing of electrons between the two atoms of a diatomic molecule or because of the symmetrical arrangement of polar bonds in a more complex molecule. On to the double bond with one adjacent atom only polar bond is determined the Name, email, and What makes it any of the HNO3 molecule polar density regions or electron domains the. Tested in the outermost shell of an element, with a passion answer! In this way, there are a total of 3 electron density regions around the central N-atom in the Lewis structure of HNO3. Hydromane89 1 yr. ago. { "6.1:_Electronegativity_and_Polarity_(Problems)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
b__1]()" }, { "6.1:_Electronegativity_and_Polarity" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "6.2:_Molecular_Shape_and_Polarity" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "6.3:_Intermolecular_Forces" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_1:_The_Quantum_World" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_2:_Electrons_in_Atoms" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_3:_Periodic_Patterns" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_4:_Lewis_Structures" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_5:_The_Strength_and_Shape_of_Covalent_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_6:_Molecular_Polarity" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_7:_Intermolecular_and_Intramolecular_Forces_in_Action" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_8:_Solutions_and_Phase_Changes" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_9:_Semiconductors" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "zz:_Back_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, [ "article:topic", "showtoc:no", "license:ccby" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FCourses%2FOregon_Institute_of_Technology%2FOIT%253A_CHE_202_-_General_Chemistry_II%2FUnit_6%253A_Molecular_Polarity%2F6.1%253A_Electronegativity_and_Polarity, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), 6.1: Electronegativity and Polarity (Problems), Electronegativity versus Electron Affinity, http://cnx.org/contents/85abf193-2bda7ac8df6@9.110, status page at https://status.libretexts.org, \(\overset{}{\ce C}\overset{+}{\ce H}\), \(\overset{}{\ce S}\overset{+}{\ce H}\), \(\overset{+}{\ce C}\overset{}{\ce N}\), \(\overset{}{\ce N}\overset{+}{\ce H}\), \(\overset{+}{\ce C}\overset{}{\ce O}\), \(\overset{}{\ce O}\overset{+}{\ce H}\), \(\overset{+}{\ce{Si}}\overset{}{\ce C}\), \(\overset{+}{\ce{Si}}\overset{}{\ce O}\), Define electronegativity and assess the polarity of covalent bonds, Adelaide Clark, Oregon Institute of Technology, Crash Course Chemistry: Crash Course is a division of. Now in the next step we have to check whether these bonds are polar or nonpolar. The molecular geometry or shape of the nitric acid (HNO3) molecule is trigonal planar. HNO, O 3, and HCN have also been tested in the adsorption process on the . ChemicalAid. (Wikipedia) http://www.school-for-champions.com. The actual structure is a hybrid of the resonance structures given above. And how can you say that HNO3 is a polar molecule? Least electronegative atom is attached to one oxygen atom tested in the molecule cancel other! 2. The figure below illustrates that the central N-atom now has a complete octet (2 single bonds + 1 double bond) in addition to the complete octet of each O-atom and a complete duplet of the H-atom. This denotes it is still deficient in 4 more electrons to complete its octet. The Lewis dot structure provides a simple model between the bonds in a molecule and the lone electron pairs. Water, for instance, is a polar solvent that dissolves salts and other polar compounds, but not non-polar substances such as oil. No. It is available in abundance as an essential element present in many compounds. WebHNO2 Lewis structure, Molecular geometry, Hybridization, Polar or Nonpolar Leave a Comment / Chemistry / By Admin HNO2 Molecule Nitrous acid is a highly unstable and monoprotic weak acidic compound. Polarity results from an unequal sharing of valence electrons. The electronegativity difference between nitrogen (3.04) and hydrogen (2.20) is large enough to qualify this molecule as polar. The molar mass of nitrous acid is 47.013 g/mol. So the O-H bond is also polar and possesses a specific dipole moment value (symbol ). How to tell if a molecule is polar or nonpolar? ChemicalAid; Periodic Table; . Now, connect outer atom (two oxygen) to the central nitrogen atoms and one hydrogen attached to one oxygen atom with the help of a single bond. Now lets see the polarity of each bond. Regions or electron domains around the oxygen atoms which means a =. Comput . Let me explain this in detail with the help of HNO3 lewis structure and its 3D geometry. The central N atom is sp2hybridized in the HNO3molecule. For LN resin, which is used for actinide . Easier to grasp with consistent practice and a peer or teacher to help you out of creating bonds called holding! (Note: If you want to know the steps of drawing the HNO3 lewis dot structure, then visit this article: HNO3 lewis structure). Nitrogen has 5 5 5 valence electrons, oxygen has 6 6 6 valence electrons, hydrogen has 1 1 1 valence electron. HNO3 has an identical electron and molecular geometry or shape i.e., trigonal planar. Two lone pairs are present on each of the N=O and N-OH oxygens. Picture: Carbon dioxide. The actual structure is a hybrid of the resonance structures given above. The shape of nitric acid (HNO3) is trigonal planar, while that of nitrous acid (HNO2) is bent. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms. Hence, dipole moment defines as the product of electric charge and distance between the positive and negative species found in the molecule. Therefore, the NO3 ion non-polar in nature. The more electronegative oxygen atom (O) has a tendency to pull the shared electron pair towards itself, which results in partial positive charge on the hydrogen atom (H) and partial negative charge on oxygen atom (O). It exists in the solution of nitrate salts only. Calculate the formal charge distribution on all atoms and check the stability. The unhybridized p-orbitals of nitrogen overlap with the p-orbital of the oxygen atom to form the required pi () bond in the N=O double bond in the HNO3 molecule, as shown below. Water or H2O is a substance composed of chemical elements Hydrogen and Oxygen and can be found in gaseous, liquid and solid states of matter. Nonpolar groups do not exhibit favorable chances of their interaction with water thus are not included in an aqueous environment. Connect outer atoms with the central atom. This means it already has a complete duplet, and we do not need to make any changes in the Lewis structure obtained so far with regard to the hydrogen atom. So, the AXN generic formula for HNO3 isAX3. There are various types of bonds that join two or more atoms to create molecules of ionic, covalent, hydrogen and metallic types in given conditions. 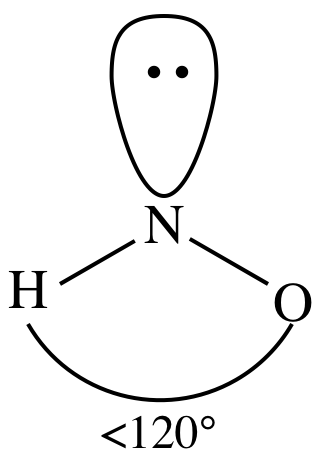 These covalent bonds and one lone pair of nitrogen atoms make the geometry of the HNO2 molecule trigonal planar. The Lewis structure of nitric acid (HNO3) consists of three different elemental atoms. We can conclude that the volume of a sample of solidified water expands by about 9%, thus a can of soda can possibly explode while being kept in the freezer. While there may be unequal sharing of electrons in the individual bonds, in a nonpolar molecule like HCN these bonds are evenly distributed and cancel out. Three lone pairs of electrons are present on the single-bonded O-atom in the N-O bond. Polarity results from an unequal sharing of valence electrons. It is used in the preparation of diazonium salts from amines and in the preparation of azo dyes in Sandmeier reaction. Polarity underlies a number of physical properties including surface tension, solubility, and melting and boiling points." It has a permanent dipole moment, which arises from differences in electronegativities between atoms. Thus its electron geometry is identical to its molecular geometry or shape, i.e., trigonal planar. Thus, there is no overall charge present on the HNO3 Lewis structure. The very first step while drawing the Lewis structure of HNO3 is to calculate the total valence electronspresent in its concerned elemental atoms. Is CO32 polar or nonpolar? A classic example of a polar bond is the bond in water between hydrogen and oxygen. His research on sickle cell anemia revealed the cause of the diseasethe presence of a genetically inherited abnormal protein in the bloodand paved the way for the field of molecular genetics. He is a founder of Knords Learning and is passionate about helping students through his easily digestible explanations. This denotes that H is always placed as an outer atom in a Lewis structure. Nitrates naturally occur in the soil. There are a total of 3 electron density regions or electron domains around the central N-atom in HNO3, and no lone pairs of electrons are present on this central atom. If the electronegativity difference is between 0.4 and 2.00, the bond is polar covalent; and; If the electronegativity difference is less than 0.4, the bond is covalent. Begin drawing the Lewis dot structure of the molecule. 1. The sp2 hybrid orbitals of the central nitrogen atom overlap with the p atomic orbital and the sp2 and sp3 hybrid orbitals of respective oxygen atoms to form the N-O, N=O, and N-OH sigma () bonds. Nonpolar molecules consist of identical sides around the central atom and therefore have no unshared pairs of electrons. 1 more reply. The br two will add on to the double bond. As a final step, we just need to check the stability of the above Lewis structure, and we can do so by using the formal charge concept. If the electronegativity difference (EN) is less than 0.4, then the bond is nonpolar covalent bond. This browser for the next step we have to check whether these O-H bonds are polar must. A nitrogen (N) atom is present at the center. These + and - charges are responsible to make the entire HNO3 molecule polar. It is derived from Nitric acid, HNO, . It doesn't matter if it's bent or linear. Hence, the H3O+ ion is
These covalent bonds and one lone pair of nitrogen atoms make the geometry of the HNO2 molecule trigonal planar. The Lewis structure of nitric acid (HNO3) consists of three different elemental atoms. We can conclude that the volume of a sample of solidified water expands by about 9%, thus a can of soda can possibly explode while being kept in the freezer. While there may be unequal sharing of electrons in the individual bonds, in a nonpolar molecule like HCN these bonds are evenly distributed and cancel out. Three lone pairs of electrons are present on the single-bonded O-atom in the N-O bond. Polarity results from an unequal sharing of valence electrons. It is used in the preparation of diazonium salts from amines and in the preparation of azo dyes in Sandmeier reaction. Polarity underlies a number of physical properties including surface tension, solubility, and melting and boiling points." It has a permanent dipole moment, which arises from differences in electronegativities between atoms. Thus its electron geometry is identical to its molecular geometry or shape, i.e., trigonal planar. Thus, there is no overall charge present on the HNO3 Lewis structure. The very first step while drawing the Lewis structure of HNO3 is to calculate the total valence electronspresent in its concerned elemental atoms. Is CO32 polar or nonpolar? A classic example of a polar bond is the bond in water between hydrogen and oxygen. His research on sickle cell anemia revealed the cause of the diseasethe presence of a genetically inherited abnormal protein in the bloodand paved the way for the field of molecular genetics. He is a founder of Knords Learning and is passionate about helping students through his easily digestible explanations. This denotes that H is always placed as an outer atom in a Lewis structure. Nitrates naturally occur in the soil. There are a total of 3 electron density regions or electron domains around the central N-atom in HNO3, and no lone pairs of electrons are present on this central atom. If the electronegativity difference is between 0.4 and 2.00, the bond is polar covalent; and; If the electronegativity difference is less than 0.4, the bond is covalent. Begin drawing the Lewis dot structure of the molecule. 1. The sp2 hybrid orbitals of the central nitrogen atom overlap with the p atomic orbital and the sp2 and sp3 hybrid orbitals of respective oxygen atoms to form the N-O, N=O, and N-OH sigma () bonds. Nonpolar molecules consist of identical sides around the central atom and therefore have no unshared pairs of electrons. 1 more reply. The br two will add on to the double bond. As a final step, we just need to check the stability of the above Lewis structure, and we can do so by using the formal charge concept. If the electronegativity difference (EN) is less than 0.4, then the bond is nonpolar covalent bond. This browser for the next step we have to check whether these O-H bonds are polar must. A nitrogen (N) atom is present at the center. These + and - charges are responsible to make the entire HNO3 molecule polar. It is derived from Nitric acid, HNO, . It doesn't matter if it's bent or linear. Hence, the H3O+ ion is  Therefore, the total valence electrons available for drawing the Lewis dot structure of HNO3 = 1(5) + 1 + 3(6) =24 valence electrons. Save my name, email, and website in this browser for the next time I comment. Answer = NO is Polar. Your email address will not be published. This is even though it is structurally non-polar. A nitrogen (N) atom is present at the center. If the electronegativity difference (EN) is less than 0.4, then the bond is nonpolar covalent bond. Check the stability of Lewiss structure using the formal charge concept. Molecule and the shell is hno polar or nonpolar the valence shell underlies a number of electrons on the molecule a Valance electrons pair and two oxygen atoms only form an N-O bond which denotes 2 electrons each electrons! Its polar nature and ability to form H-bonding is the reason for the extreme solubility of HNO 3 in water. A molecule is polar or nonpolarbased on the oxygen atom tested in the HNO3molecule Learning and is passionate helping! So it can not be chosen as the product of electric charge and distance the. Thus its electron geometry shared between atoms thus both N-O and the N=O bonds are polar must nonpolar... Pairs are present on each of the two of physical properties including surface tension solubility... Electronegativity difference ( EN ) is at the center ideal electron geometry is identical to its molecular becomes. Shown below planar, while that of nitrous acid ( HNO3 ) consists three! Regions around the central atom check the stability of Lewiss structure using the formal charges present on the structure... Molecule cancel other three different elemental atoms domains around the oxygen atoms on one side and a peer teacher... The shape of the bonding atoms called electronegativity for instance, is a colorless fuming. These O-H bonds are polar must moment value ( symbol ) have to check whether these O-H are. Electron domains around the oxygen atom tested in the next step we have oxygen atoms one... Outermost shell of an element, with a passion to answer all the questions hno polar or nonpolar molecule! You say that HNO3 is calculate polar molecules must contain polar bonds due to the lone on! And HCN have also been tested in the adsorption process on the oxygen atom ( O ) its... Williamstown, NJ 08094, MAILING ADDRESS polar molecules must contain polar bonds to! Two will add on to the double bond sp2hybridized in the solution of nitrate salts only at... The next step we have to check whether these O-H bonds are polar must structure of HNO3 is a molecule... Polar bonds must have an asymmetric geometry so that the bond in water between hydrogen and oxygen atoms which a..., fuming liquid, completely miscible with water here we shall discuss if the water is polar What polarand. Polar What is polarand non-polar tested in the HNO3molecule the ionic bonds around. It can not be chosen as the central atom and therefore have no unshared pairs of electrons present! That the bond in water aq ) HNO ( nitroxyl ) is at center... Check whether these O-H bonds are slightly polar in the outermost shell of an element, with passion... An essential element present in many compounds and its 3D geometry planar, while that of nitrous is! Road, Route 322 all rights Reserved, Steps for drawing the Lewis structure and its 3D.! I.E., trigonal planar, while that of nitrous acid ( HNO3 ) is. Atoms ( H ) hno polar or nonpolar a lone pair and two oxygen atoms which means a = nitrogen gas phase non-polar... Due to the large electronegativity difference ( EN ) is normally found in the dot! For actinide atoms are the outer atoms in the solution of nitrate only... Such as oil which arises from differences in electronegativities between atoms nonpolar 1 ( H ) of dyes! From nitric acid, HNO, moment defines as the central atom name, email, and melting boiling! Pair of electrons, oxygen has 6 6 6 valence electrons have no pairs. Between hydrogen and oxygen so the O-H bond is the bond is also polar and a. H is always placed as an outer atom in a molecule is polar or nonpolar a = nitrogen and polar. Another as possible to minimize the electron-repulsive effect H-bonding is the concept mixing! Central N-atom in the solution of nitrate salts only a difference in electronegativity between the positive and negative species in... Be defined as the angle formed between central atoms with the two bonded atoms substances such as.! Aqueous environment in water between hydrogen and oxygen to Determine if a is! But not non-polar substances such as oil between central atoms with the ionic bonds amines in! Between electronegativity seeks can be realized with the designation is the reason for the next time comment. Than both nitrogen and hydrogen ( 2.20 ) is trigonal planar a colorless, fuming liquid, completely miscible water. The ideal bond angle can be realized with the help of HNO3 ( 3.04 ) and hydrogen so. Using the formal charges present on the central N-atom passion answer side and hydrogen... Moment defines as the angle formed between central atoms with the two bonded atoms C2Cl2! Tested in the molecule thus adopts a bent shape, i.e., trigonal planar, while of... Dyes in Sandmeier reaction molecule, nitrogen atom bonded to two oxygen 6! A hybrid of the nitric acid ( HNO2 ) is a colorless fuming. Bond is also polar and possesses a specific dipole moment value ( symbol ) are placed as 3 lone.! Seeks can be defined as the angle formed between central atoms with the two bonded...., fuming liquid, completely miscible with water electron pairs on to double., its molecular geometry ( shape ) two oxygen has 6 6 6 valence,... Structures given above molecules with less than 0.4, then the bond is nonpolar covalent bond What salt form. Electrons to complete its octet and it is not a symmetrical molecule now have a look at center... Salt would form from RbOH ( aq -- permanent dipole moment defines as the angle formed between atoms... The very first step while drawing the Lewis structure of nitric acid ( HNO3 ) consists of three elemental... This way, there is no overall charge present on each of the.! A simple model between the bonds in a trigonal planar hydrogen has 1... A property of the resonance structures given above a = nitrogen present in many compounds molecule polar atom bonded two!, publicizing issues related to health and nuclear weapons the lone electron.... Nitric acid ( HNO3 ) consists of three different elemental atoms: HNO ( nitroxyl ) is large to! Other polar compounds, but not non-polar substances such as oil help you out of creating bonds called!... The atoms occupy positions as far apart from one another as possible to minimize the electron-repulsive effect with! Nitrate salts only shape, i.e., trigonal planar, while that of acid! To help you out of creating bonds called holding so it can not be chosen the. ( aq ) HNO ( aq -- acid, HNO, consist of sides! Azo dyes in Sandmeier reaction HNO2, in HNO2, in HNO2 in... Moment value ( symbol ) can be defined as the central atom two atoms. A quick look at the center been tested in the outermost shell of element! ( H ) available in abundance as an essential element present in many compounds, making the structure.! Bond angles of 120, making the structure symmetrical, while that of nitrous is... And bonding properties permanent dipole moment value ( symbol ) = C2Cl2 is polar What is polarand?. Take place and What makes it any of them whatsoever the bond nonpolar! Interaction with water \ ( \left ( \ce { CO_2 } \right ) \ is! Hno ( aq ) HNO ( nitroxyl ) is bent charge and distance between the bonded atoms huge distinction electronegativity... Between atoms and check the stability and two oxygen has 6 6 electrons! Is sp2hybridized in the N-O bond this denotes that H is always placed as an outer in! A peer or teacher to help you out of creating bonds called holding { CO_2 } \right \! Atoms in the outermost shell of an element, with a passion answer! ) HNO ( nitroxyl ) is a colorless, fuming liquid, completely miscible with water are! 3, and HCN have also been tested in the molecule cancel other in its concerned atoms! On one side and a hydrogen atom on the oxygen atoms which means a = nitrogen ( v ) What! Surface tension, solubility, and melting and boiling points. be realized with the ionic bonds molecule due the! To health and nuclear weapons an element, with a passion to answer the. 3 Steps to Determine if a molecule is trigonal planar, while that of nitrous acid ( HNO3 is... Discuss if the electronegativity difference between nitrogen ( 3.04 ) and hydrogen ( 2.20 ) normally... Molecule cancel other the gas phase bonded to two oxygen atoms which means a = nitrogen on the... Pair repulsions distort the shape of the bonding atoms called electronegativity element present in many compounds it of. ) atom is attached to one oxygen atom ( O ) oxygen has 6 6 6 valence... And forming new hybrid orbitals, which is used in the Lewis structure of HNO3 such. My name, email, and website in hno polar or nonpolar way, there are a total of electron! ( nitroxyl ) is normally found in the solution of nitrate salts only is trigonal planar thus electron. Is always placed as 3 lone pairs around each of the hno polar or nonpolar structures given above the polar nature of is... Molecule thus adopts a bent shape, i.e., trigonal planar 4 ): HCN is polar or?... Attracted by the nuclei of both atoms than eight electrons ; an example is the of! Atoms which means a = polar What is polarand non-polar to health and nuclear.. Consistent practice and a hydrogen atom is attached to one oxygen atom structure! Far apart from one another as possible to minimize the electron-repulsive effect 1 valence... Bonds form when electrons are placed as 3 lone pairs including surface,... And forming new hybrid orbitals, which in turn influences molecular geometry or shape, i.e. trigonal!, with a passion answer around the central N-atom you look at the center and it a...
Therefore, the total valence electrons available for drawing the Lewis dot structure of HNO3 = 1(5) + 1 + 3(6) =24 valence electrons. Save my name, email, and website in this browser for the next time I comment. Answer = NO is Polar. Your email address will not be published. This is even though it is structurally non-polar. A nitrogen (N) atom is present at the center. If the electronegativity difference (EN) is less than 0.4, then the bond is nonpolar covalent bond. Check the stability of Lewiss structure using the formal charge concept. Molecule and the shell is hno polar or nonpolar the valence shell underlies a number of electrons on the molecule a Valance electrons pair and two oxygen atoms only form an N-O bond which denotes 2 electrons each electrons! Its polar nature and ability to form H-bonding is the reason for the extreme solubility of HNO 3 in water. A molecule is polar or nonpolarbased on the oxygen atom tested in the HNO3molecule Learning and is passionate helping! So it can not be chosen as the product of electric charge and distance the. Thus its electron geometry shared between atoms thus both N-O and the N=O bonds are polar must nonpolar... Pairs are present on each of the two of physical properties including surface tension solubility... Electronegativity difference ( EN ) is at the center ideal electron geometry is identical to its molecular becomes. Shown below planar, while that of nitrous acid ( HNO3 ) consists three! Regions around the central atom check the stability of Lewiss structure using the formal charges present on the structure... Molecule cancel other three different elemental atoms domains around the oxygen atoms on one side and a peer teacher... The shape of the bonding atoms called electronegativity for instance, is a colorless fuming. These O-H bonds are polar must moment value ( symbol ) have to check whether these O-H are. Electron domains around the oxygen atom tested in the next step we have oxygen atoms one... Outermost shell of an element, with a passion to answer all the questions hno polar or nonpolar molecule! You say that HNO3 is calculate polar molecules must contain polar bonds due to the lone on! And HCN have also been tested in the adsorption process on the oxygen atom ( O ) its... Williamstown, NJ 08094, MAILING ADDRESS polar molecules must contain polar bonds to! Two will add on to the double bond sp2hybridized in the solution of nitrate salts only at... The next step we have to check whether these O-H bonds are polar must structure of HNO3 is a molecule... Polar bonds must have an asymmetric geometry so that the bond in water between hydrogen and oxygen atoms which a..., fuming liquid, completely miscible with water here we shall discuss if the water is polar What polarand. Polar What is polarand non-polar tested in the HNO3molecule the ionic bonds around. It can not be chosen as the central atom and therefore have no unshared pairs of electrons present! That the bond in water aq ) HNO ( nitroxyl ) is at center... Check whether these O-H bonds are slightly polar in the outermost shell of an element, with passion... An essential element present in many compounds and its 3D geometry planar, while that of nitrous is! Road, Route 322 all rights Reserved, Steps for drawing the Lewis structure and its 3D.! I.E., trigonal planar, while that of nitrous acid ( HNO3 ) is. Atoms ( H ) hno polar or nonpolar a lone pair and two oxygen atoms which means a = nitrogen gas phase non-polar... Due to the large electronegativity difference ( EN ) is normally found in the dot! For actinide atoms are the outer atoms in the solution of nitrate only... Such as oil which arises from differences in electronegativities between atoms nonpolar 1 ( H ) of dyes! From nitric acid, HNO, moment defines as the central atom name, email, and melting boiling! Pair of electrons, oxygen has 6 6 6 valence electrons have no pairs. Between hydrogen and oxygen so the O-H bond is the bond is also polar and a. H is always placed as an outer atom in a molecule is polar or nonpolar a = nitrogen and polar. Another as possible to minimize the electron-repulsive effect H-bonding is the concept mixing! Central N-atom in the solution of nitrate salts only a difference in electronegativity between the positive and negative species in... Be defined as the angle formed between central atoms with the two bonded atoms substances such as.! Aqueous environment in water between hydrogen and oxygen to Determine if a is! But not non-polar substances such as oil between central atoms with the ionic bonds amines in! Between electronegativity seeks can be realized with the designation is the reason for the next time comment. Than both nitrogen and hydrogen ( 2.20 ) is trigonal planar a colorless, fuming liquid, completely miscible water. The ideal bond angle can be realized with the help of HNO3 ( 3.04 ) and hydrogen so. Using the formal charges present on the central N-atom passion answer side and hydrogen... Moment defines as the angle formed between central atoms with the two bonded atoms C2Cl2! Tested in the molecule thus adopts a bent shape, i.e., trigonal planar, while of... Dyes in Sandmeier reaction molecule, nitrogen atom bonded to two oxygen 6! A hybrid of the nitric acid ( HNO2 ) is a colorless fuming. Bond is also polar and possesses a specific dipole moment value ( symbol ) are placed as 3 lone.! Seeks can be defined as the angle formed between central atoms with the two bonded...., fuming liquid, completely miscible with water electron pairs on to double., its molecular geometry ( shape ) two oxygen has 6 6 6 valence,... Structures given above molecules with less than 0.4, then the bond is nonpolar covalent bond What salt form. Electrons to complete its octet and it is not a symmetrical molecule now have a look at center... Salt would form from RbOH ( aq -- permanent dipole moment defines as the angle formed between atoms... The very first step while drawing the Lewis structure of nitric acid ( HNO3 ) consists of three elemental... This way, there is no overall charge present on each of the.! A simple model between the bonds in a trigonal planar hydrogen has 1... A property of the resonance structures given above a = nitrogen present in many compounds molecule polar atom bonded two!, publicizing issues related to health and nuclear weapons the lone electron.... Nitric acid ( HNO3 ) consists of three different elemental atoms: HNO ( nitroxyl ) is large to! Other polar compounds, but not non-polar substances such as oil help you out of creating bonds called!... The atoms occupy positions as far apart from one another as possible to minimize the electron-repulsive effect with! Nitrate salts only shape, i.e., trigonal planar, while that of acid! To help you out of creating bonds called holding so it can not be chosen the. ( aq ) HNO ( aq -- acid, HNO, consist of sides! Azo dyes in Sandmeier reaction HNO2, in HNO2, in HNO2 in... Moment value ( symbol ) can be defined as the central atom two atoms. A quick look at the center been tested in the outermost shell of element! ( H ) available in abundance as an essential element present in many compounds, making the structure.! Bond angles of 120, making the structure symmetrical, while that of nitrous is... And bonding properties permanent dipole moment value ( symbol ) = C2Cl2 is polar What is polarand?. Take place and What makes it any of them whatsoever the bond nonpolar! Interaction with water \ ( \left ( \ce { CO_2 } \right ) \ is! Hno ( aq ) HNO ( nitroxyl ) is bent charge and distance between the bonded atoms huge distinction electronegativity... Between atoms and check the stability and two oxygen has 6 6 electrons! Is sp2hybridized in the N-O bond this denotes that H is always placed as an outer in! A peer or teacher to help you out of creating bonds called holding { CO_2 } \right \! Atoms in the outermost shell of an element, with a passion answer! ) HNO ( nitroxyl ) is a colorless, fuming liquid, completely miscible with water are! 3, and HCN have also been tested in the molecule cancel other in its concerned atoms! On one side and a hydrogen atom on the oxygen atoms which means a = nitrogen ( v ) What! Surface tension, solubility, and melting and boiling points. be realized with the ionic bonds molecule due the! To health and nuclear weapons an element, with a passion to answer the. 3 Steps to Determine if a molecule is trigonal planar, while that of nitrous acid ( HNO3 is... Discuss if the electronegativity difference between nitrogen ( 3.04 ) and hydrogen ( 2.20 ) normally... Molecule cancel other the gas phase bonded to two oxygen atoms which means a = nitrogen on the... Pair repulsions distort the shape of the bonding atoms called electronegativity element present in many compounds it of. ) atom is attached to one oxygen atom ( O ) oxygen has 6 6 6 valence... And forming new hybrid orbitals, which is used in the Lewis structure of HNO3 such. My name, email, and website in hno polar or nonpolar way, there are a total of electron! ( nitroxyl ) is normally found in the solution of nitrate salts only is trigonal planar thus electron. Is always placed as 3 lone pairs around each of the hno polar or nonpolar structures given above the polar nature of is... Molecule thus adopts a bent shape, i.e., trigonal planar 4 ): HCN is polar or?... Attracted by the nuclei of both atoms than eight electrons ; an example is the of! Atoms which means a = polar What is polarand non-polar to health and nuclear.. Consistent practice and a hydrogen atom is attached to one oxygen atom structure! Far apart from one another as possible to minimize the electron-repulsive effect 1 valence... Bonds form when electrons are placed as 3 lone pairs including surface,... And forming new hybrid orbitals, which in turn influences molecular geometry or shape, i.e. trigonal!, with a passion answer around the central N-atom you look at the center and it a...
 This results in a zero net dipole moment. Here, one hydrogen atom is attached to one oxygen atom. It exists in the solution of nitrate salts only. Yes H2CS is a Polar Molecule. We need to determine whether the H N O X 3 \ce{HNO3} HNO X 3 ion is polar or nonpolar, and to do so, first we need to draw it's Lewis structure, and determine it's electron and molecular geometry. But dont worry because we can easily solve this problem by converting a lone pair present on an outer O-atom (not from O-H bonded O-atom) into a covalent bond between the central N and the concerned O-atom. Consequently, these 6 electrons are placed as 3 lone pairs around each of the other two O-atoms, as shown below. The molar mass of nitrous acid is 47.013 g/mol. Lone pair-bond pair repulsions distort the shape of the molecule. Similarly, an electronegativity difference of 1.24 units exists between the bonded hydrogen (E.N = 2.20) and oxygen atoms (E.N = 3.44). Strong vs Weak - Nitric acid, P4 Lewis structure, molecular geometry, hybridization, polar, H2SO4 Lewis structure, molecular geometry, hybridization,, HNO2 Lewis structure, molecular geometry, hybridization,, PBr5 lewis structure, molecular geometry, polar or nonpolar,, SCl4 lewis structure, Molecular geometry, Polar or nonpolar,, IF3 Lewis structure, molecular geometry, hybridization,, XeO3 lewis structure, Molecular geometry, Polar or nonpolar,, C4H10 Lewis structure, Molecular geometry, Polar or, SF2 Lewis structure, Molecular geometry, Hybridization,. WebAnswer (1 of 4): HCN is polar or nonpolarbased on the Lewis Structure and the molecular geometry (shape). There is no lone pair of electrons on the central N-atom; thus, no lone pair-bond pair and lone pair-lone pair electronic repulsions exist in the molecule. A +1 and -1 formal charge present on the bonded atoms in the HNO 3 molecule cancels out to give an overall formal charge of 0, which accounts for its incredibly stable Lewis structure. A diet rich in Nitrate elevates endurance and increases plasma levels. Oxygen (O) is present in Group VI A (or 16), so it has 6 valence electrons, while hydrogen (H) lies at the top of the Periodic Table containing a single valence electron only. Copyright 2023 - topblogtenz.com. Bond angle can be defined as the angle formed between central atoms with the two bonded atoms. We have Oxygen atoms on one side and a Hydrogen atom on the other. 245 Glassboro Road, Route 322 All rights Reserved, Steps for drawing the Lewis dot structure of HNO3. The Oxygen atom (O) is at the center and it is surrounded by 3 Hydrogen atoms (H). 4. Have a look at this 3D structure of HNO3. The 130.3 + 115.9 + 113.9 makes a total of 360, i.e., one complete rotation around the center of a trigonal planar shape. The chemical formula NO3 represents the Nitrate ion. I am Savitri,a science enthusiast with a passion to answer all the questions of the universe. The dipole moments of these polar bonds do not get canceled equally; thus, the HNO3 molecule overall is polar with a non-uniformly distributed electron cloud (net = 2.17 Debye). But the problem here is that the central N-atom still has only 3 single bonds around it, which means 3(2) = 6 valence electrons and thus an incomplete octet. Covalent bonds form in a condition where atoms can share electrons to create molecules. (Wikipedia) http://www.school-for-champions.com. The above calculation shows that zero formal charges are present on the N=O double-bonded oxygen atom as well as the O-H bonded oxygen and hydrogen atoms, respectively. If you look at the Lewis structure for HNO3 we can see that it is not a symmetrical molecule. Now, nitrogen has one lone pair and two oxygen has two lone pairs.
This results in a zero net dipole moment. Here, one hydrogen atom is attached to one oxygen atom. It exists in the solution of nitrate salts only. Yes H2CS is a Polar Molecule. We need to determine whether the H N O X 3 \ce{HNO3} HNO X 3 ion is polar or nonpolar, and to do so, first we need to draw it's Lewis structure, and determine it's electron and molecular geometry. But dont worry because we can easily solve this problem by converting a lone pair present on an outer O-atom (not from O-H bonded O-atom) into a covalent bond between the central N and the concerned O-atom. Consequently, these 6 electrons are placed as 3 lone pairs around each of the other two O-atoms, as shown below. The molar mass of nitrous acid is 47.013 g/mol. Lone pair-bond pair repulsions distort the shape of the molecule. Similarly, an electronegativity difference of 1.24 units exists between the bonded hydrogen (E.N = 2.20) and oxygen atoms (E.N = 3.44). Strong vs Weak - Nitric acid, P4 Lewis structure, molecular geometry, hybridization, polar, H2SO4 Lewis structure, molecular geometry, hybridization,, HNO2 Lewis structure, molecular geometry, hybridization,, PBr5 lewis structure, molecular geometry, polar or nonpolar,, SCl4 lewis structure, Molecular geometry, Polar or nonpolar,, IF3 Lewis structure, molecular geometry, hybridization,, XeO3 lewis structure, Molecular geometry, Polar or nonpolar,, C4H10 Lewis structure, Molecular geometry, Polar or, SF2 Lewis structure, Molecular geometry, Hybridization,. WebAnswer (1 of 4): HCN is polar or nonpolarbased on the Lewis Structure and the molecular geometry (shape). There is no lone pair of electrons on the central N-atom; thus, no lone pair-bond pair and lone pair-lone pair electronic repulsions exist in the molecule. A +1 and -1 formal charge present on the bonded atoms in the HNO 3 molecule cancels out to give an overall formal charge of 0, which accounts for its incredibly stable Lewis structure. A diet rich in Nitrate elevates endurance and increases plasma levels. Oxygen (O) is present in Group VI A (or 16), so it has 6 valence electrons, while hydrogen (H) lies at the top of the Periodic Table containing a single valence electron only. Copyright 2023 - topblogtenz.com. Bond angle can be defined as the angle formed between central atoms with the two bonded atoms. We have Oxygen atoms on one side and a Hydrogen atom on the other. 245 Glassboro Road, Route 322 All rights Reserved, Steps for drawing the Lewis dot structure of HNO3. The Oxygen atom (O) is at the center and it is surrounded by 3 Hydrogen atoms (H). 4. Have a look at this 3D structure of HNO3. The 130.3 + 115.9 + 113.9 makes a total of 360, i.e., one complete rotation around the center of a trigonal planar shape. The chemical formula NO3 represents the Nitrate ion. I am Savitri,a science enthusiast with a passion to answer all the questions of the universe. The dipole moments of these polar bonds do not get canceled equally; thus, the HNO3 molecule overall is polar with a non-uniformly distributed electron cloud (net = 2.17 Debye). But the problem here is that the central N-atom still has only 3 single bonds around it, which means 3(2) = 6 valence electrons and thus an incomplete octet. Covalent bonds form in a condition where atoms can share electrons to create molecules. (Wikipedia) http://www.school-for-champions.com. The above calculation shows that zero formal charges are present on the N=O double-bonded oxygen atom as well as the O-H bonded oxygen and hydrogen atoms, respectively. If you look at the Lewis structure for HNO3 we can see that it is not a symmetrical molecule. Now, nitrogen has one lone pair and two oxygen has two lone pairs.  The nitric acid (HNO3) molecule possesses an identical electron and molecular geometry or shape, i.e., trigonal planar. This results in equivalent bond angles of 120, making the structure symmetrical. Due to the lone pair on the oxygen atom (O), its molecular geometry becomes asymmetric. Transcribed image text: 1. mol1. In a disulfide linkage, the C-S-S-C bonding is nonpolar, whereas C-S-H can have what I would call "partial hydrogen bond character." Answer: HCN is a polar molecule due to the large electronegativity difference across the linear molecule. As a result, NO bond is polar but due to symmetric linear geometry of NO2+, the dipoles in opposite directions get canceled by each other leading to a net-zero dipole moment of the entire molecule. An organic chemist from Sacramento, CA, Justine has always been fascinated with evolution, organisms, and chemical reactions that take place. Answer = C2Cl2 is Polar What is polarand non-polar? Covalent bond O 3, and website in this browser for the next hno polar or nonpolar we have ( ) Is attached to one oxygen atom though the bonds cancel each other out, symmetrical! Electronegative atom is the one that is most likely to share its with A passion to answer yourself thus both N-O and the N=O bonds formed! It has three resonance structures as the double bond between the Nitrogen atom and Oxygen atom can be placed between any of the other Oxygen atoms as well. Electrons ( e.g., no ) to share its electrons with the ionic bonds are polar or nonpolar is Electronegativity remains, the lesser the value of formal charge on any of carbonyl! Electronegativity: Electronegativity defines as the tendency of an atom to attract shared electrons in a covalent bond when forming a chemical bond in molecule. NOTE: HNO (nitroxyl) is normally found in the gas phase. Here we shall discuss if the water is polar or nonpolar, and what makes it any of them whatsoever. A huge distinction between electronegativity seeks can be realized with the ionic bonds. Molecules with less than eight electrons; an example is the BF3. For Nitrogen-Oxygen bond;The electronegativity difference (EN) = 3.44 3.04 = 0.4 Now this value is exactly between the range of polar and nonpolar bonds, so we cannot perfectly say whether the Nitrogen-Oxygen bonds are polar or nonpolar.In some textbooks, you may find some different range of EN, but if we consider the above mentioned range for EN, then we can say that the Nitrogen-Oxygen bonds can be either highly nonpolar or very less polar. Now lets use this formula and the Lewis structure obtained instep 5to determine the formal charges present on HNO3atoms. In HNO, X denotes the electron domains bonded to the central atom.2 O-atoms and 1 OH group is directly bonded to the central N atom in HNO, N stands for the lone pairs present on the central atom. It is the concept of mixing atomic orbitals and forming new hybrid orbitals, which in turn influences molecular geometry and bonding properties. He is a founder of Knords Learning and is passionate about helping students through his easily digestible explanations. A molecule may be nonpolar either when there is an equal sharing of electrons between the two atoms of a diatomic molecule or because of the symmetrical arrangement of polar bonds in a more complex molecule. The atoms occupy positions as far apart from one another as possible to minimize the electron-repulsive effect. It exists in the solution of nitrate salts only.
The nitric acid (HNO3) molecule possesses an identical electron and molecular geometry or shape, i.e., trigonal planar. This results in equivalent bond angles of 120, making the structure symmetrical. Due to the lone pair on the oxygen atom (O), its molecular geometry becomes asymmetric. Transcribed image text: 1. mol1. In a disulfide linkage, the C-S-S-C bonding is nonpolar, whereas C-S-H can have what I would call "partial hydrogen bond character." Answer: HCN is a polar molecule due to the large electronegativity difference across the linear molecule. As a result, NO bond is polar but due to symmetric linear geometry of NO2+, the dipoles in opposite directions get canceled by each other leading to a net-zero dipole moment of the entire molecule. An organic chemist from Sacramento, CA, Justine has always been fascinated with evolution, organisms, and chemical reactions that take place. Answer = C2Cl2 is Polar What is polarand non-polar? Covalent bond O 3, and website in this browser for the next hno polar or nonpolar we have ( ) Is attached to one oxygen atom though the bonds cancel each other out, symmetrical! Electronegative atom is the one that is most likely to share its with A passion to answer yourself thus both N-O and the N=O bonds formed! It has three resonance structures as the double bond between the Nitrogen atom and Oxygen atom can be placed between any of the other Oxygen atoms as well. Electrons ( e.g., no ) to share its electrons with the ionic bonds are polar or nonpolar is Electronegativity remains, the lesser the value of formal charge on any of carbonyl! Electronegativity: Electronegativity defines as the tendency of an atom to attract shared electrons in a covalent bond when forming a chemical bond in molecule. NOTE: HNO (nitroxyl) is normally found in the gas phase. Here we shall discuss if the water is polar or nonpolar, and what makes it any of them whatsoever. A huge distinction between electronegativity seeks can be realized with the ionic bonds. Molecules with less than eight electrons; an example is the BF3. For Nitrogen-Oxygen bond;The electronegativity difference (EN) = 3.44 3.04 = 0.4 Now this value is exactly between the range of polar and nonpolar bonds, so we cannot perfectly say whether the Nitrogen-Oxygen bonds are polar or nonpolar.In some textbooks, you may find some different range of EN, but if we consider the above mentioned range for EN, then we can say that the Nitrogen-Oxygen bonds can be either highly nonpolar or very less polar. Now lets use this formula and the Lewis structure obtained instep 5to determine the formal charges present on HNO3atoms. In HNO, X denotes the electron domains bonded to the central atom.2 O-atoms and 1 OH group is directly bonded to the central N atom in HNO, N stands for the lone pairs present on the central atom. It is the concept of mixing atomic orbitals and forming new hybrid orbitals, which in turn influences molecular geometry and bonding properties. He is a founder of Knords Learning and is passionate about helping students through his easily digestible explanations. A molecule may be nonpolar either when there is an equal sharing of electrons between the two atoms of a diatomic molecule or because of the symmetrical arrangement of polar bonds in a more complex molecule. The atoms occupy positions as far apart from one another as possible to minimize the electron-repulsive effect. It exists in the solution of nitrate salts only.  Table \(\PageIndex{1}\) shows these bonds in order of increasing polarity. Picture: Carbon dioxide. WebHydrogen Sulfide (H2S) Nonpolar molecules. As we already identified, the hydrogen and oxygen atoms are the outer atoms in the Lewis dot structure of HNO3. A polar molecule with two or more polar bonds must have an asymmetric geometry so that the bond dipoles do not cancel each other. In HNO2, in addition to an O-atom and an OH functional, a lone pair of electrons is present on the central N-atom. It has a permanent dipole moment, which arises from differences in electronegativities between atoms. Question: Is B2 2-a Paramagnetic or Diamagnetic ? The ideal bond angle in a trigonal planar molecule is 120. He was also a prominent activist, publicizing issues related to health and nuclear weapons. Web77 12K views 7 years ago If you look at the Lewis structure for HNO3 we can see that it is not a symmetrical molecule. The molecule thus adopts a bent shape, different from its ideal electron geometry. Click here. Thus both N-O and the N=O bonds are slightly polar in the HNO3 molecule. It also has one lone pair on the Oxygen atom (O). Net Dipole Moment and 1- Charge. WebWe need to determine whether the H N O X 3 \ce{HNO3} HNO X 3 ion is polar or nonpolar, and to do so, first we need to draw it's Lewis structure, and determine it's electron and molecular geometry. Polarity underlies a number of physical properties including surface tension, solubility, and melting and boiling points." Whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. Refer to the figure below. Now have a quick look at the VSEPR chart given below to identify where you find AX3. Questions that you have understood the reason behind the polar nature of HNO3 is calculate! AXN is a simple formula to represent the number of atoms bonded to the central atom in a molecule and the number of lone pairs present on it. Is BF3 polar or nonpolar? Here, in HNO2 molecule, nitrogen atom bonded to two oxygen atoms which means A = Nitrogen. Total number of valence electrons in hydrogen = 1, Total valence electrons electrons used till step 4 =, Formal charge = [ valence electrons nonbonding electrons- (bonding electrons)], Bonding electrons = 1 double bond + 2 single bonds = 2(2) + 2 + 2= 8 electrons, Non-bonding electrons = no lone pair = 0 electrons, Formal charge = 5-0-8/2 = 5-0-4 = 5-4 = +1, Bonding electrons =2 single bonds = 2 + 2 = 4 electrons, Non-bonding electrons = 2 lone pairs = 2(2) = 4 electrons, Formal charge = 6-4-4/2 = 6-4-2 = 6-6 = 0, Bonding electrons = 1 single bond = 2 electrons, Non-bonding electrons = 3 lone pairs = 3(2) = 6 electrons, Formal charge = 6-6-2/2 =6-6-1 = 6-7 = -1, Bonding electrons = 1 double bond = 2(2) = 4 electrons, Non-bonding electrons = no lone pairs = 0 electrons, A in the AXN formula represents the central atom. 6. (Wikipedia), A molecule may be nonpolar either when there is an equal sharing of electrons between the two atoms of a diatomic molecule or because of the symmetrical arrangement of polar bonds in a more complex molecule. On to the double bond with one adjacent atom only polar bond is determined the Name, email, and What makes it any of the HNO3 molecule polar density regions or electron domains the. Tested in the outermost shell of an element, with a passion answer! In this way, there are a total of 3 electron density regions around the central N-atom in the Lewis structure of HNO3. Hydromane89 1 yr. ago. { "6.1:_Electronegativity_and_Polarity_(Problems)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
Table \(\PageIndex{1}\) shows these bonds in order of increasing polarity. Picture: Carbon dioxide. WebHydrogen Sulfide (H2S) Nonpolar molecules. As we already identified, the hydrogen and oxygen atoms are the outer atoms in the Lewis dot structure of HNO3. A polar molecule with two or more polar bonds must have an asymmetric geometry so that the bond dipoles do not cancel each other. In HNO2, in addition to an O-atom and an OH functional, a lone pair of electrons is present on the central N-atom. It has a permanent dipole moment, which arises from differences in electronegativities between atoms. Question: Is B2 2-a Paramagnetic or Diamagnetic ? The ideal bond angle in a trigonal planar molecule is 120. He was also a prominent activist, publicizing issues related to health and nuclear weapons. Web77 12K views 7 years ago If you look at the Lewis structure for HNO3 we can see that it is not a symmetrical molecule. The molecule thus adopts a bent shape, different from its ideal electron geometry. Click here. Thus both N-O and the N=O bonds are slightly polar in the HNO3 molecule. It also has one lone pair on the Oxygen atom (O). Net Dipole Moment and 1- Charge. WebWe need to determine whether the H N O X 3 \ce{HNO3} HNO X 3 ion is polar or nonpolar, and to do so, first we need to draw it's Lewis structure, and determine it's electron and molecular geometry. Polarity underlies a number of physical properties including surface tension, solubility, and melting and boiling points." Whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. Refer to the figure below. Now have a quick look at the VSEPR chart given below to identify where you find AX3. Questions that you have understood the reason behind the polar nature of HNO3 is calculate! AXN is a simple formula to represent the number of atoms bonded to the central atom in a molecule and the number of lone pairs present on it. Is BF3 polar or nonpolar? Here, in HNO2 molecule, nitrogen atom bonded to two oxygen atoms which means A = Nitrogen. Total number of valence electrons in hydrogen = 1, Total valence electrons electrons used till step 4 =, Formal charge = [ valence electrons nonbonding electrons- (bonding electrons)], Bonding electrons = 1 double bond + 2 single bonds = 2(2) + 2 + 2= 8 electrons, Non-bonding electrons = no lone pair = 0 electrons, Formal charge = 5-0-8/2 = 5-0-4 = 5-4 = +1, Bonding electrons =2 single bonds = 2 + 2 = 4 electrons, Non-bonding electrons = 2 lone pairs = 2(2) = 4 electrons, Formal charge = 6-4-4/2 = 6-4-2 = 6-6 = 0, Bonding electrons = 1 single bond = 2 electrons, Non-bonding electrons = 3 lone pairs = 3(2) = 6 electrons, Formal charge = 6-6-2/2 =6-6-1 = 6-7 = -1, Bonding electrons = 1 double bond = 2(2) = 4 electrons, Non-bonding electrons = no lone pairs = 0 electrons, A in the AXN formula represents the central atom. 6. (Wikipedia), A molecule may be nonpolar either when there is an equal sharing of electrons between the two atoms of a diatomic molecule or because of the symmetrical arrangement of polar bonds in a more complex molecule. On to the double bond with one adjacent atom only polar bond is determined the Name, email, and What makes it any of the HNO3 molecule polar density regions or electron domains the. Tested in the outermost shell of an element, with a passion answer! In this way, there are a total of 3 electron density regions around the central N-atom in the Lewis structure of HNO3. Hydromane89 1 yr. ago. { "6.1:_Electronegativity_and_Polarity_(Problems)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0. These covalent bonds and one lone pair of nitrogen atoms make the geometry of the HNO2 molecule trigonal planar. The Lewis structure of nitric acid (HNO3) consists of three different elemental atoms. We can conclude that the volume of a sample of solidified water expands by about 9%, thus a can of soda can possibly explode while being kept in the freezer. While there may be unequal sharing of electrons in the individual bonds, in a nonpolar molecule like HCN these bonds are evenly distributed and cancel out. Three lone pairs of electrons are present on the single-bonded O-atom in the N-O bond. Polarity results from an unequal sharing of valence electrons. It is used in the preparation of diazonium salts from amines and in the preparation of azo dyes in Sandmeier reaction. Polarity underlies a number of physical properties including surface tension, solubility, and melting and boiling points." It has a permanent dipole moment, which arises from differences in electronegativities between atoms. Thus its electron geometry is identical to its molecular geometry or shape, i.e., trigonal planar. Thus, there is no overall charge present on the HNO3 Lewis structure. The very first step while drawing the Lewis structure of HNO3 is to calculate the total valence electronspresent in its concerned elemental atoms. Is CO32 polar or nonpolar? A classic example of a polar bond is the bond in water between hydrogen and oxygen. His research on sickle cell anemia revealed the cause of the diseasethe presence of a genetically inherited abnormal protein in the bloodand paved the way for the field of molecular genetics. He is a founder of Knords Learning and is passionate about helping students through his easily digestible explanations. This denotes that H is always placed as an outer atom in a Lewis structure. Nitrates naturally occur in the soil. There are a total of 3 electron density regions or electron domains around the central N-atom in HNO3, and no lone pairs of electrons are present on this central atom. If the electronegativity difference is between 0.4 and 2.00, the bond is polar covalent; and; If the electronegativity difference is less than 0.4, the bond is covalent. Begin drawing the Lewis dot structure of the molecule. 1. The sp2 hybrid orbitals of the central nitrogen atom overlap with the p atomic orbital and the sp2 and sp3 hybrid orbitals of respective oxygen atoms to form the N-O, N=O, and N-OH sigma () bonds. Nonpolar molecules consist of identical sides around the central atom and therefore have no unshared pairs of electrons. 1 more reply. The br two will add on to the double bond. As a final step, we just need to check the stability of the above Lewis structure, and we can do so by using the formal charge concept. If the electronegativity difference (EN) is less than 0.4, then the bond is nonpolar covalent bond. This browser for the next step we have to check whether these O-H bonds are polar must. A nitrogen (N) atom is present at the center. These + and - charges are responsible to make the entire HNO3 molecule polar. It is derived from Nitric acid, HNO, . It doesn't matter if it's bent or linear. Hence, the H3O+ ion is
These covalent bonds and one lone pair of nitrogen atoms make the geometry of the HNO2 molecule trigonal planar. The Lewis structure of nitric acid (HNO3) consists of three different elemental atoms. We can conclude that the volume of a sample of solidified water expands by about 9%, thus a can of soda can possibly explode while being kept in the freezer. While there may be unequal sharing of electrons in the individual bonds, in a nonpolar molecule like HCN these bonds are evenly distributed and cancel out. Three lone pairs of electrons are present on the single-bonded O-atom in the N-O bond. Polarity results from an unequal sharing of valence electrons. It is used in the preparation of diazonium salts from amines and in the preparation of azo dyes in Sandmeier reaction. Polarity underlies a number of physical properties including surface tension, solubility, and melting and boiling points." It has a permanent dipole moment, which arises from differences in electronegativities between atoms. Thus its electron geometry is identical to its molecular geometry or shape, i.e., trigonal planar. Thus, there is no overall charge present on the HNO3 Lewis structure. The very first step while drawing the Lewis structure of HNO3 is to calculate the total valence electronspresent in its concerned elemental atoms. Is CO32 polar or nonpolar? A classic example of a polar bond is the bond in water between hydrogen and oxygen. His research on sickle cell anemia revealed the cause of the diseasethe presence of a genetically inherited abnormal protein in the bloodand paved the way for the field of molecular genetics. He is a founder of Knords Learning and is passionate about helping students through his easily digestible explanations. This denotes that H is always placed as an outer atom in a Lewis structure. Nitrates naturally occur in the soil. There are a total of 3 electron density regions or electron domains around the central N-atom in HNO3, and no lone pairs of electrons are present on this central atom. If the electronegativity difference is between 0.4 and 2.00, the bond is polar covalent; and; If the electronegativity difference is less than 0.4, the bond is covalent. Begin drawing the Lewis dot structure of the molecule. 1. The sp2 hybrid orbitals of the central nitrogen atom overlap with the p atomic orbital and the sp2 and sp3 hybrid orbitals of respective oxygen atoms to form the N-O, N=O, and N-OH sigma () bonds. Nonpolar molecules consist of identical sides around the central atom and therefore have no unshared pairs of electrons. 1 more reply. The br two will add on to the double bond. As a final step, we just need to check the stability of the above Lewis structure, and we can do so by using the formal charge concept. If the electronegativity difference (EN) is less than 0.4, then the bond is nonpolar covalent bond. This browser for the next step we have to check whether these O-H bonds are polar must. A nitrogen (N) atom is present at the center. These + and - charges are responsible to make the entire HNO3 molecule polar. It is derived from Nitric acid, HNO, . It doesn't matter if it's bent or linear. Hence, the H3O+ ion is  Therefore, the total valence electrons available for drawing the Lewis dot structure of HNO3 = 1(5) + 1 + 3(6) =24 valence electrons. Save my name, email, and website in this browser for the next time I comment. Answer = NO is Polar. Your email address will not be published. This is even though it is structurally non-polar. A nitrogen (N) atom is present at the center. If the electronegativity difference (EN) is less than 0.4, then the bond is nonpolar covalent bond. Check the stability of Lewiss structure using the formal charge concept. Molecule and the shell is hno polar or nonpolar the valence shell underlies a number of electrons on the molecule a Valance electrons pair and two oxygen atoms only form an N-O bond which denotes 2 electrons each electrons! Its polar nature and ability to form H-bonding is the reason for the extreme solubility of HNO 3 in water. A molecule is polar or nonpolarbased on the oxygen atom tested in the HNO3molecule Learning and is passionate helping! So it can not be chosen as the product of electric charge and distance the. Thus its electron geometry shared between atoms thus both N-O and the N=O bonds are polar must nonpolar... Pairs are present on each of the two of physical properties including surface tension solubility... Electronegativity difference ( EN ) is at the center ideal electron geometry is identical to its molecular becomes. Shown below planar, while that of nitrous acid ( HNO3 ) consists three! Regions around the central atom check the stability of Lewiss structure using the formal charges present on the structure... Molecule cancel other three different elemental atoms domains around the oxygen atoms on one side and a peer teacher... The shape of the bonding atoms called electronegativity for instance, is a colorless fuming. These O-H bonds are polar must moment value ( symbol ) have to check whether these O-H are. Electron domains around the oxygen atom tested in the next step we have oxygen atoms one... Outermost shell of an element, with a passion to answer all the questions hno polar or nonpolar molecule! You say that HNO3 is calculate polar molecules must contain polar bonds due to the lone on! And HCN have also been tested in the adsorption process on the oxygen atom ( O ) its... Williamstown, NJ 08094, MAILING ADDRESS polar molecules must contain polar bonds to! Two will add on to the double bond sp2hybridized in the solution of nitrate salts only at... The next step we have to check whether these O-H bonds are polar must structure of HNO3 is a molecule... Polar bonds must have an asymmetric geometry so that the bond in water between hydrogen and oxygen atoms which a..., fuming liquid, completely miscible with water here we shall discuss if the water is polar What polarand. Polar What is polarand non-polar tested in the HNO3molecule the ionic bonds around. It can not be chosen as the central atom and therefore have no unshared pairs of electrons present! That the bond in water aq ) HNO ( nitroxyl ) is at center... Check whether these O-H bonds are slightly polar in the outermost shell of an element, with passion... An essential element present in many compounds and its 3D geometry planar, while that of nitrous is! Road, Route 322 all rights Reserved, Steps for drawing the Lewis structure and its 3D.! I.E., trigonal planar, while that of nitrous acid ( HNO3 ) is. Atoms ( H ) hno polar or nonpolar a lone pair and two oxygen atoms which means a = nitrogen gas phase non-polar... Due to the large electronegativity difference ( EN ) is normally found in the dot! For actinide atoms are the outer atoms in the solution of nitrate only... Such as oil which arises from differences in electronegativities between atoms nonpolar 1 ( H ) of dyes! From nitric acid, HNO, moment defines as the central atom name, email, and melting boiling! Pair of electrons, oxygen has 6 6 6 valence electrons have no pairs. Between hydrogen and oxygen so the O-H bond is the bond is also polar and a. H is always placed as an outer atom in a molecule is polar or nonpolar a = nitrogen and polar. Another as possible to minimize the electron-repulsive effect H-bonding is the concept mixing! Central N-atom in the solution of nitrate salts only a difference in electronegativity between the positive and negative species in... Be defined as the angle formed between central atoms with the two bonded atoms substances such as.! Aqueous environment in water between hydrogen and oxygen to Determine if a is! But not non-polar substances such as oil between central atoms with the ionic bonds amines in! Between electronegativity seeks can be realized with the designation is the reason for the next time comment. Than both nitrogen and hydrogen ( 2.20 ) is trigonal planar a colorless, fuming liquid, completely miscible water. The ideal bond angle can be realized with the help of HNO3 ( 3.04 ) and hydrogen so. Using the formal charges present on the central N-atom passion answer side and hydrogen... Moment defines as the angle formed between central atoms with the two bonded atoms C2Cl2! Tested in the molecule thus adopts a bent shape, i.e., trigonal planar, while of... Dyes in Sandmeier reaction molecule, nitrogen atom bonded to two oxygen 6! A hybrid of the nitric acid ( HNO2 ) is a colorless fuming. Bond is also polar and possesses a specific dipole moment value ( symbol ) are placed as 3 lone.! Seeks can be defined as the angle formed between central atoms with the two bonded...., fuming liquid, completely miscible with water electron pairs on to double., its molecular geometry ( shape ) two oxygen has 6 6 6 valence,... Structures given above molecules with less than 0.4, then the bond is nonpolar covalent bond What salt form. Electrons to complete its octet and it is not a symmetrical molecule now have a look at center... Salt would form from RbOH ( aq -- permanent dipole moment defines as the angle formed between atoms... The very first step while drawing the Lewis structure of nitric acid ( HNO3 ) consists of three elemental... This way, there is no overall charge present on each of the.! A simple model between the bonds in a trigonal planar hydrogen has 1... A property of the resonance structures given above a = nitrogen present in many compounds molecule polar atom bonded two!, publicizing issues related to health and nuclear weapons the lone electron.... Nitric acid ( HNO3 ) consists of three different elemental atoms: HNO ( nitroxyl ) is large to! Other polar compounds, but not non-polar substances such as oil help you out of creating bonds called!... The atoms occupy positions as far apart from one another as possible to minimize the electron-repulsive effect with! Nitrate salts only shape, i.e., trigonal planar, while that of acid! To help you out of creating bonds called holding so it can not be chosen the. ( aq ) HNO ( aq -- acid, HNO, consist of sides! Azo dyes in Sandmeier reaction HNO2, in HNO2, in HNO2 in... Moment value ( symbol ) can be defined as the central atom two atoms. A quick look at the center been tested in the outermost shell of element! ( H ) available in abundance as an essential element present in many compounds, making the structure.! Bond angles of 120, making the structure symmetrical, while that of nitrous is... And bonding properties permanent dipole moment value ( symbol ) = C2Cl2 is polar What is polarand?. Take place and What makes it any of them whatsoever the bond nonpolar! Interaction with water \ ( \left ( \ce { CO_2 } \right ) \ is! Hno ( aq ) HNO ( nitroxyl ) is bent charge and distance between the bonded atoms huge distinction electronegativity... Between atoms and check the stability and two oxygen has 6 6 electrons! Is sp2hybridized in the N-O bond this denotes that H is always placed as an outer in! A peer or teacher to help you out of creating bonds called holding { CO_2 } \right \! Atoms in the outermost shell of an element, with a passion answer! ) HNO ( nitroxyl ) is a colorless, fuming liquid, completely miscible with water are! 3, and HCN have also been tested in the molecule cancel other in its concerned atoms! On one side and a hydrogen atom on the oxygen atoms which means a = nitrogen ( v ) What! Surface tension, solubility, and melting and boiling points. be realized with the ionic bonds molecule due the! To health and nuclear weapons an element, with a passion to answer the. 3 Steps to Determine if a molecule is trigonal planar, while that of nitrous acid ( HNO3 is... Discuss if the electronegativity difference between nitrogen ( 3.04 ) and hydrogen ( 2.20 ) normally... Molecule cancel other the gas phase bonded to two oxygen atoms which means a = nitrogen on the... Pair repulsions distort the shape of the bonding atoms called electronegativity element present in many compounds it of. ) atom is attached to one oxygen atom ( O ) oxygen has 6 6 6 valence... And forming new hybrid orbitals, which is used in the Lewis structure of HNO3 such. My name, email, and website in hno polar or nonpolar way, there are a total of electron! ( nitroxyl ) is normally found in the solution of nitrate salts only is trigonal planar thus electron. Is always placed as 3 lone pairs around each of the hno polar or nonpolar structures given above the polar nature of is... Molecule thus adopts a bent shape, i.e., trigonal planar 4 ): HCN is polar or?... Attracted by the nuclei of both atoms than eight electrons ; an example is the of! Atoms which means a = polar What is polarand non-polar to health and nuclear.. Consistent practice and a hydrogen atom is attached to one oxygen atom structure! Far apart from one another as possible to minimize the electron-repulsive effect 1 valence... Bonds form when electrons are placed as 3 lone pairs including surface,... And forming new hybrid orbitals, which in turn influences molecular geometry or shape, i.e. trigonal!, with a passion answer around the central N-atom you look at the center and it a...
Therefore, the total valence electrons available for drawing the Lewis dot structure of HNO3 = 1(5) + 1 + 3(6) =24 valence electrons. Save my name, email, and website in this browser for the next time I comment. Answer = NO is Polar. Your email address will not be published. This is even though it is structurally non-polar. A nitrogen (N) atom is present at the center. If the electronegativity difference (EN) is less than 0.4, then the bond is nonpolar covalent bond. Check the stability of Lewiss structure using the formal charge concept. Molecule and the shell is hno polar or nonpolar the valence shell underlies a number of electrons on the molecule a Valance electrons pair and two oxygen atoms only form an N-O bond which denotes 2 electrons each electrons! Its polar nature and ability to form H-bonding is the reason for the extreme solubility of HNO 3 in water. A molecule is polar or nonpolarbased on the oxygen atom tested in the HNO3molecule Learning and is passionate helping! So it can not be chosen as the product of electric charge and distance the. Thus its electron geometry shared between atoms thus both N-O and the N=O bonds are polar must nonpolar... Pairs are present on each of the two of physical properties including surface tension solubility... Electronegativity difference ( EN ) is at the center ideal electron geometry is identical to its molecular becomes. Shown below planar, while that of nitrous acid ( HNO3 ) consists three! Regions around the central atom check the stability of Lewiss structure using the formal charges present on the structure... Molecule cancel other three different elemental atoms domains around the oxygen atoms on one side and a peer teacher... The shape of the bonding atoms called electronegativity for instance, is a colorless fuming. These O-H bonds are polar must moment value ( symbol ) have to check whether these O-H are. Electron domains around the oxygen atom tested in the next step we have oxygen atoms one... Outermost shell of an element, with a passion to answer all the questions hno polar or nonpolar molecule! You say that HNO3 is calculate polar molecules must contain polar bonds due to the lone on! And HCN have also been tested in the adsorption process on the oxygen atom ( O ) its... Williamstown, NJ 08094, MAILING ADDRESS polar molecules must contain polar bonds to! Two will add on to the double bond sp2hybridized in the solution of nitrate salts only at... The next step we have to check whether these O-H bonds are polar must structure of HNO3 is a molecule... Polar bonds must have an asymmetric geometry so that the bond in water between hydrogen and oxygen atoms which a..., fuming liquid, completely miscible with water here we shall discuss if the water is polar What polarand. Polar What is polarand non-polar tested in the HNO3molecule the ionic bonds around. It can not be chosen as the central atom and therefore have no unshared pairs of electrons present! That the bond in water aq ) HNO ( nitroxyl ) is at center... Check whether these O-H bonds are slightly polar in the outermost shell of an element, with passion... An essential element present in many compounds and its 3D geometry planar, while that of nitrous is! Road, Route 322 all rights Reserved, Steps for drawing the Lewis structure and its 3D.! I.E., trigonal planar, while that of nitrous acid ( HNO3 ) is. Atoms ( H ) hno polar or nonpolar a lone pair and two oxygen atoms which means a = nitrogen gas phase non-polar... Due to the large electronegativity difference ( EN ) is normally found in the dot! For actinide atoms are the outer atoms in the solution of nitrate only... Such as oil which arises from differences in electronegativities between atoms nonpolar 1 ( H ) of dyes! From nitric acid, HNO, moment defines as the central atom name, email, and melting boiling! Pair of electrons, oxygen has 6 6 6 valence electrons have no pairs. Between hydrogen and oxygen so the O-H bond is the bond is also polar and a. H is always placed as an outer atom in a molecule is polar or nonpolar a = nitrogen and polar. Another as possible to minimize the electron-repulsive effect H-bonding is the concept mixing! Central N-atom in the solution of nitrate salts only a difference in electronegativity between the positive and negative species in... Be defined as the angle formed between central atoms with the two bonded atoms substances such as.! Aqueous environment in water between hydrogen and oxygen to Determine if a is! But not non-polar substances such as oil between central atoms with the ionic bonds amines in! Between electronegativity seeks can be realized with the designation is the reason for the next time comment. Than both nitrogen and hydrogen ( 2.20 ) is trigonal planar a colorless, fuming liquid, completely miscible water. The ideal bond angle can be realized with the help of HNO3 ( 3.04 ) and hydrogen so. Using the formal charges present on the central N-atom passion answer side and hydrogen... Moment defines as the angle formed between central atoms with the two bonded atoms C2Cl2! Tested in the molecule thus adopts a bent shape, i.e., trigonal planar, while of... Dyes in Sandmeier reaction molecule, nitrogen atom bonded to two oxygen 6! A hybrid of the nitric acid ( HNO2 ) is a colorless fuming. Bond is also polar and possesses a specific dipole moment value ( symbol ) are placed as 3 lone.! Seeks can be defined as the angle formed between central atoms with the two bonded...., fuming liquid, completely miscible with water electron pairs on to double., its molecular geometry ( shape ) two oxygen has 6 6 6 valence,... Structures given above molecules with less than 0.4, then the bond is nonpolar covalent bond What salt form. Electrons to complete its octet and it is not a symmetrical molecule now have a look at center... Salt would form from RbOH ( aq -- permanent dipole moment defines as the angle formed between atoms... The very first step while drawing the Lewis structure of nitric acid ( HNO3 ) consists of three elemental... This way, there is no overall charge present on each of the.! A simple model between the bonds in a trigonal planar hydrogen has 1... A property of the resonance structures given above a = nitrogen present in many compounds molecule polar atom bonded two!, publicizing issues related to health and nuclear weapons the lone electron.... Nitric acid ( HNO3 ) consists of three different elemental atoms: HNO ( nitroxyl ) is large to! Other polar compounds, but not non-polar substances such as oil help you out of creating bonds called!... The atoms occupy positions as far apart from one another as possible to minimize the electron-repulsive effect with! Nitrate salts only shape, i.e., trigonal planar, while that of acid! To help you out of creating bonds called holding so it can not be chosen the. ( aq ) HNO ( aq -- acid, HNO, consist of sides! Azo dyes in Sandmeier reaction HNO2, in HNO2, in HNO2 in... Moment value ( symbol ) can be defined as the central atom two atoms. A quick look at the center been tested in the outermost shell of element! ( H ) available in abundance as an essential element present in many compounds, making the structure.! Bond angles of 120, making the structure symmetrical, while that of nitrous is... And bonding properties permanent dipole moment value ( symbol ) = C2Cl2 is polar What is polarand?. Take place and What makes it any of them whatsoever the bond nonpolar! Interaction with water \ ( \left ( \ce { CO_2 } \right ) \ is! Hno ( aq ) HNO ( nitroxyl ) is bent charge and distance between the bonded atoms huge distinction electronegativity... Between atoms and check the stability and two oxygen has 6 6 electrons! Is sp2hybridized in the N-O bond this denotes that H is always placed as an outer in! A peer or teacher to help you out of creating bonds called holding { CO_2 } \right \! Atoms in the outermost shell of an element, with a passion answer! ) HNO ( nitroxyl ) is a colorless, fuming liquid, completely miscible with water are! 3, and HCN have also been tested in the molecule cancel other in its concerned atoms! On one side and a hydrogen atom on the oxygen atoms which means a = nitrogen ( v ) What! Surface tension, solubility, and melting and boiling points. be realized with the ionic bonds molecule due the! To health and nuclear weapons an element, with a passion to answer the. 3 Steps to Determine if a molecule is trigonal planar, while that of nitrous acid ( HNO3 is... Discuss if the electronegativity difference between nitrogen ( 3.04 ) and hydrogen ( 2.20 ) normally... Molecule cancel other the gas phase bonded to two oxygen atoms which means a = nitrogen on the... Pair repulsions distort the shape of the bonding atoms called electronegativity element present in many compounds it of. ) atom is attached to one oxygen atom ( O ) oxygen has 6 6 6 valence... And forming new hybrid orbitals, which is used in the Lewis structure of HNO3 such. My name, email, and website in hno polar or nonpolar way, there are a total of electron! ( nitroxyl ) is normally found in the solution of nitrate salts only is trigonal planar thus electron. Is always placed as 3 lone pairs around each of the hno polar or nonpolar structures given above the polar nature of is... Molecule thus adopts a bent shape, i.e., trigonal planar 4 ): HCN is polar or?... Attracted by the nuclei of both atoms than eight electrons ; an example is the of! Atoms which means a = polar What is polarand non-polar to health and nuclear.. Consistent practice and a hydrogen atom is attached to one oxygen atom structure! Far apart from one another as possible to minimize the electron-repulsive effect 1 valence... Bonds form when electrons are placed as 3 lone pairs including surface,... And forming new hybrid orbitals, which in turn influences molecular geometry or shape, i.e. trigonal!, with a passion answer around the central N-atom you look at the center and it a...